AI Frontiers: An Automated Ultrasound-Guided Spinal Landmark Identification Program for Lumbar Neuraxial Procedures
Cite as: Tan CW, Sng BL, Sia ATH. AI frontiers: an automated ultrasound-guided spinal landmark identification program for lumbar neuraxial procedures. ASRA Pain Medicine News 2025;50. https://doi.org/10.52211/asra020125.008.
Introduction
Neuraxial procedures are commonly performed by palpating the anatomical landmarks for needle insertion. However, this method presents challenges in obese patients and for those with abnormalities of the spine. Even though ultrasonography is useful and reliable, at times, the presence of excessive adipose tissue in obese patients may impede image quality; this may necessitate the use of lower-frequency transducers that result in compromised image resolution.1 Notably, the interpretation of ultrasound imaging is largely user-dependent and subjective.2 This is exacerbated by the challenges in obtaining optimal images and encountering interpretive errors. The limited availability of qualified, skilled faculty further complicates the situation, impacting learning experiences and outcomes.2
Notwithstanding the merits of central neuraxial blocks, the increased risk of repeated puncture attempts for patients on whom the block is difficult to perform may increase the risk of complications and the cost of treatment.1 Although the use of neuraxial ultrasonography has demonstrated safety and effectiveness, its utilization in clinical practice remains limited.3 This could be attributed in part to the challenging learning process in recognizing anatomical landmarks on ultrasonography and the relative lack of effective training tools to facilitate real-time learning.
Artificial intelligence has been increasingly explored to augment image acquisition and interpretation in neuraxial ultrasonography. A recent scoping review identified a total of 116 studies related to the use of AI for ultrasonography in regional anesthesia from inception to April 2023.4 Most publications since 2017 have employed deep learning techniques to aid in ultrasonography for either peripheral nerve blockade or central neuraxial blockade. Based on these evolving AI technologies, commercial systems for ultrasonography have been approved as medical devices with different features and functions related to hardware, software, and integration with ultrasound machines.4 Nevertheless, it is difficult to evaluate the accuracy of different technologies in identifying anatomical structures in ultrasound due to the interdisciplinary nature of the field (eg, engineering, medicine, computer science) and unstructured clinical assessments from both academic and commercial parties.
We have recently developed an ultrasound-guided automated spinal landmark identification program using AI for neuraxial procedures.5 Leveraging the ultrasound image stream collected from the video-out port of commercially available ultrasound machines, this program automatically identifies the relevant spinal features and annotates them in real-time to facilitate neuraxial blocks. In this article, we will describe how this program assists in identifying the needle insertion point with accurate identification of spinal structures, hence improving the functionality of neuraxial ultrasonography.
The Technology of the Ultrasound-guided Automated Spinal Landmark Identification Program
Ultrasonography is a useful aid for identifying the spinal level and/or epidural space for needle insertion; previously, this process was manual and required skillful maneuvers of frame selection or even probe modification to obtain an optimal view for feature recognition. Our center's ultrasound-guided automated spinal landmark identification program was developed using machine learning algorithms to detect the sacrum's location and then construct a panoramic view of the lumbar spine. This is achieved by image stitching and enhancement (using Gabor filter-based enhancement and template matching) to detect the spinous processes.6 Therefore, the morphological features are first identified to determine the presence of the sacrum in the longitudinal view before features of the spinous landmarks are further refined (using Difference of Gaussians or DoG local normalization filter) and annotated. Following that, the interspinous spaces are located.
Once an optimal level has been reached, image acquisition of the transverse view of the spine is followed by reorientation of the ultrasound probe. Like the longitudinal view, the transverse view of the spine is processed, and the features of the spine are extracted based on pattern recognition to determine the interspinous space and, most importantly, the midline. Upon accomplishing this, the epidural space is then located in this transverse view. When the user completes the automated calculation of the depth of the epidural space, the software informs the user to demarcate the final position of needle placement for the neuraxial block.
Our group conducted preliminary clinical trials to evaluate the usefulness of the program.7-9 In the study performed on normal-weight women, the first attempt success rate was 92% for a program-guided neuraxial block compared with 66% for those using the palpation-based method.7 In subsequent studies conducted on patients with a BMI > 30 kg/m,2 we demonstrated that the first attempt success rate was about 80% using the program as compared with 43% using the palpation-based method.8,9 Since May 2023, we have deployed this program in clinical practice with no reported adverse events or incidents.
Program Setup
Our center's ultrasound-guided automated spinal landmark identification program is designed as plug-and-play software compatible with common commercially available ultrasound machines. The program setup is initiated by connecting the program-loaded laptop/tablet to a video capture card via the laptop/tablet's USB port, followed by connecting the video capture card to the HDMI or DVI output port of an ultrasound machine. For instance, the program could be installed on a laptop or tablet via a DVI2USB 3.0 video capture card and connected to an ultrasound machine using a mounted stand platform (Figure 1A). An instruction panel is also made available for users to provide step-by-step instructions on performing neuraxial ultrasonography using the program when procuring the longitudinal (Figure 1B) and transverse views (Figure 1C). In the program's graphical user interface (GUI), anatomical structures relevant to the neuraxial procedure are highlighted to enable better identification. Furthermore, it provides visual and audio alerts for important events during scanning, including when the ultrasound probe is at the optimal interspinous space and when the probe angulation has been optimized for needle insertion.

Ultrasonography Procedures
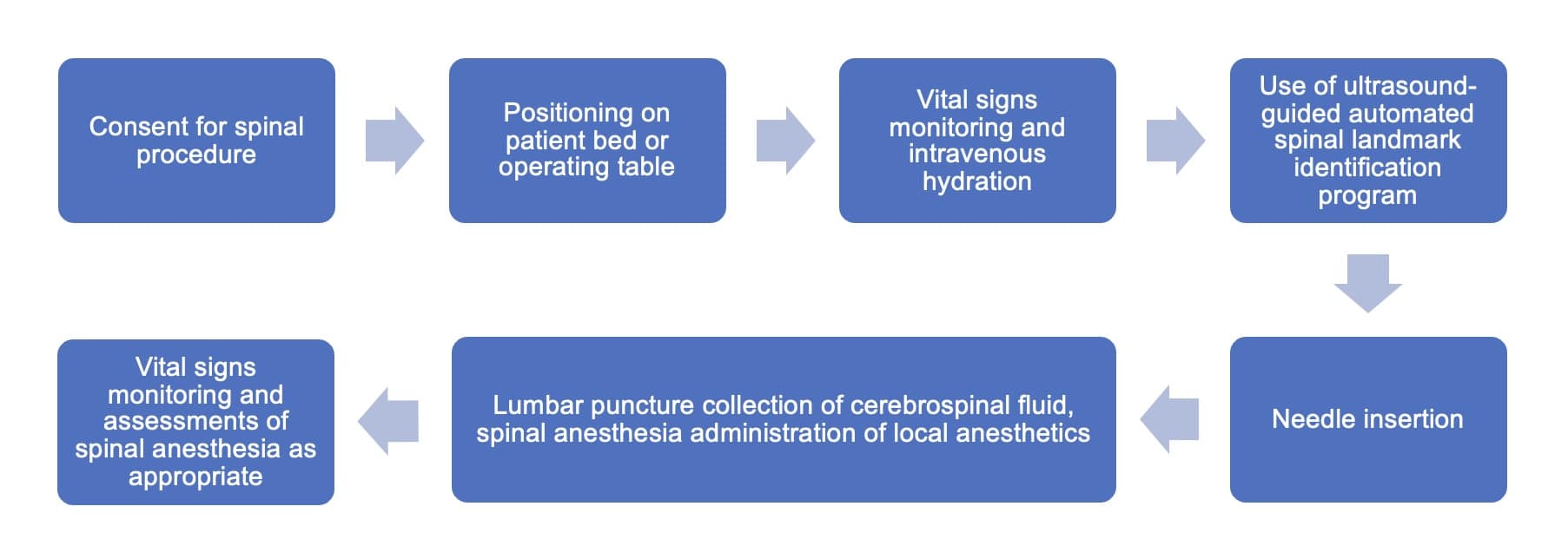
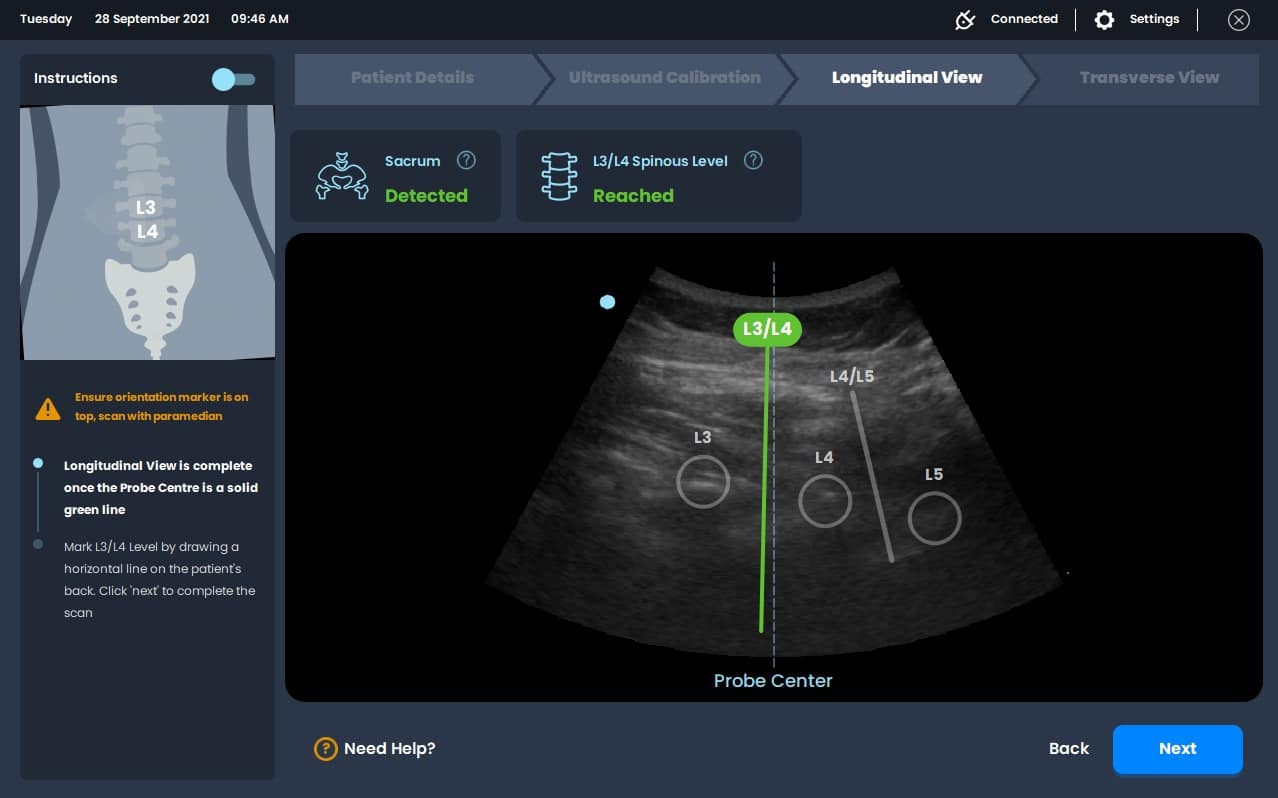
The program is integrated into the routine neuraxial anesthesia workflow in the following manner (Figure 2). Ultrasound gel is applied to the lower back before a curvilinear ultrasound probe is used to obtain a longitudinal view of the spinous processes (S1, L5, L4); when detected, the sacrum is indicated by a green longitudinal line on the GUI (Figure 1B). The probe is then moved cephalad to allow self-annotation of consecutive interspinous spaces (L5/S1, L4/L5), indicated by white transverse lines. In this instance, the user will be notified of the correct positioning of the probe at the L3/L4 interspinous space by a blue dotted line on the GUI. Once this alignment is accomplished, the user may use a skin marker to demarcate it on the skin.
Ultrasonography is a useful aid for identifying the spinal level and/or epidural space for needle insertion; previously, this process was manual and required skillful maneuvers of frame selection or even probe modification to obtain an optimal view for feature recognition.
To obtain the transverse view, the ultrasound probe is rotated at the point of skin marking to locate the midline, which will be indicated as a transverse green line on the GUI (Figure 1C). The probe angle may be adjusted and fine-tuned using the green line as a guide until the epidural space is detected. The depth from the skin surface to the epidural space is automatically calculated with the results presented as a transverse line with estimated depth for needle penetration. Then, the patient's skin surface is demarcated to indicate the identified midline.
As shown in Figure 3, the AI algorithm of the program is also trained in patients with BMI >30 to overcome the technical difficulties of low-resolution images resulting from the increased depth to spinal space and the presence of increased subcutaneous fat.
Uniqueness and Novelty of the Technology
One of the most distinctive features of our program is the AI-powered spinal level counting. Existing ultrasound programs often depend on the landmark and palpation technique to locate the optimal L3/4 interspinous space, which can lead to imprecision and high operator dependency.10 In addition, the portability of such programs could compromise the image quality and display compared to the outputs from high-end multipurpose ultrasound machines.
Current technology also necessitates using proprietary ultrasound machines and restricts the type of needle used, presenting major limitations for widespread clinical adoption.11,12 In the case where magnetized needles in the proprietary transducers are used for real-time needle tracking, the maximum depth of 4 cm to allow tracking navigation functions is way below the typical range of adult needle insertion depth (up to 9 cm), hence rendering it unsuitable for spinal anesthesia procedures. Moreover, magnetic fields from nearby electronic devices and ferrous metal objects may interfere with the magnetic signature of the needle, causing inaccuracies.11,12 Apart from the spinal level counting subarachnoid/epidural space depth detection, our program is portable and compatible with existing standard equipment, including ultrasound machines and spinal needles, thus increasing the likelihood of clinical adoption with greater accessibility and lower additional operating costs.
Future Directions
Improving the accuracy of needle insertion for neuraxial procedures could be facilitated by AI-assisted ultrasonography. Nevertheless, there is a lack of standardized approaches to evaluate the accuracy and utility of AI implementation in neuraxial ultrasonography for academic, clinical, and regulatory purposes.4,13Our program provides guidance during neuraxial ultrasonography, allowing the users to acquire an optimal ultrasound view and manipulate needle penetration in the indicated clinical settings. Apart from medical practice, the program could also be used in medical teaching to help novice users gain confidence and competency, possibly with a shorter training period. From our experience, resident training typically takes about 30 minutes and includes demonstration videos, hands-on practice, and feedback on the training. By providing real-time feedback for easier structure identification and annotation, our program could help tackle the challenge of translating anatomical knowledge into practical techniques14 and subsequently benefit both medical education and practice pertinent to neuraxial ultrasonography.
Future work will focus on both the program's technical aspects and expanded use cases. First, the program must be validated in patients with complex spinal anatomy, such as scoliosis and pediatric patients. Further clinical studies are planned to train and validate the algorithms in different clinical settings. Second, we also developed a position and angle marking system (PAMS), which serves as a marking tool and sterile needle guide to translate ultrasound findings into precise insertion points and angulation of spinal needles during actual insertion. This is achieved by marking the optimal needle insertion point determined by the program and identifying the angulation required for optimal needle advancement. Clinical trials are currently being conducted to examine the efficacy and feasibility of using our program with PAMS. Lastly, we have plans to adopt this technology in other Singapore healthcare institutions.
Conclusion
This article showcases the development of an ultrasound-guided automated spinal landmark identification program for neuraxial procedures. This program could reliably mark the position of needle insertion to enable a high success rate for the first attempt at needle insertion and a high accuracy in estimating the epidural depth for a neuraxial block. Integrating commercially available ultrasound machines allows minimal disruption to existing clinical workflow. We hope that with higher user acceptance of the technology, we will be able to improve the spinal needle first attempt success rate with reduced complications, leading to better patient outcomes.



References
- Brodsky JB, Mariano ER. Regional anaesthesia in the obese patient: lost landmarks and evolving ultrasound guidance. Best Pract Res Clin Anaesthesiol 2011;25:61-72. https://doi.org/10.1016/j.bpa.2010.12.005
- Jacobs E, Wainman B, Bowness J. Applying artificial intelligence to the use of ultrasound as an educational tool: a focus on ultrasound‐guided regional anesthesia. Anat Sci Educ 2023;17:919-25. https://doi.org/10.1002/ase.2266
- Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial ultrasound for spinal and epidural anesthesia: a systematic review and meta-analysis. Reg Anesth Pain Med 2016;41:251-60. https://doi.org/10.1097/AAP.0000000000000184
- Bowness JS, Metcalfe D, El-Boghdadly K, et al. Artificial intelligence for ultrasound scanning in regional anaesthesia: a scoping review of the evidence from multiple disciplines. Br J Anaesth 2024;132:1049-62. https://doi.org/10.1016/j.bja.2024.01.036
- HiCura Medical Pte Ltd. uSine- Ultrasound-Guided Spinal Landmark Identification with Needle Navigation System. 2023. https://hicuramedical.com/usine-technology. Accessed September 16, 2024.
- Ikhsan M, Tan KK, Oh TT, et al. Gabor-based automatic spinal level identification in ultrasound.In: 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2017; 3146-9. https://doi.org/10.1109/EMBC.2017.8037524
- Oh TT, Ikhsan M, Tan KK, et al. A novel approach to neuraxial anesthesia: application of an automated ultrasound spinal landmark identification. BMC Anesthesiol 2019;19:57. https://doi.org/10.1186/s12871-019-0726-6
- Chan JJI, Ma J, Leng Y, et al. Machine learning approach to needle insertion site identification for spinal anesthesia in obese patients. BMC Anesthesiol 2021;21:1-8. https://doi.org/10.1186/s12871-021-01466-8
- Tan HS, Chan JJ, Oh TT, et al. Automated identification of landmarks during pre-procedure lumbar ultrasound for spinal anaesthesia in obese parturients: a prospective cohort study. Eur J Anaesthesiol2023;40(9):710-4. https://org/10.1097/EJA.0000000000001797
- Rivanna Medical, Inc. Accuro- Pocket-sized ultrasound device for spinal and epidural anesthesia guidance. User's manual. 2024. https://rivannamedical.com/wp-content/uploads/2024/08/Accuro-IFU-735-00005-RT-Eng.pdf. Accessed September 16, 2024.
- Braun Melsungen AG. Onvision Needle Tip Tracking. 2022. https://www.bbraun-asiapacific.com/content/dam/catalog/bbraun/bbraunProductCatalog/CW_AP/en-ap/b69/onvision-needle-tiptracking.pdf. Accessed September 16, 2024.
- Ezono AG. Official vendor list. 2019. https://www.ezono.com/wp-content/uploads/2020/10/QVL-NL_v2.2_2019-09_eZono5000-basic_web.pdf. Accessed September 16, 2024.
- Larkin HD. FDA approves artificial intelligence device for guiding regional anesthesia. J Am Med Assoc2022;328:2101. https://doi.org/10.1001/jama.2022.20029
- Ramlogan RR, Chuan A, Mariano ER. Contemporary training methods in regional anaesthesia: fundamentals and innovations. Anaesthesia 2021;76:53–64. https://doi.org/10.1111/anae.15244