Pros and Cons: The Transdiscal Approach to Celiac Plexus Block With or Without Neurolysis
Cite as: Craven JM, Cohen SP. Pros and cons: the transdiscal approach to celiac plexus block with or without neurolysis. ASRA Pain Medicine News 2025;50. https://doi.org/10.52211/asra020125.010.
Pros & Cons
Introduction
Celiac plexus blocks have long been used to treat visceral nociceptive pain with the technique first described by Kappis in 1919.1 The technique can be performed via several distinct transcutaneous approaches, which include retrocrural, anterocrural, and various iterations of these two main approaches, including bilateral splanchnic nerve block, transaortic, bilateral transcrural, or transdiscal as well as by endoscopic ultrasound guidance. The optimal technique depends on the patient’s anatomy, and reviewing the patient’s most recent CT scan or MRI is imperative to assess the location of tumor burden, vascular structures, kidneys, or pleura, which may be transected by needle placement. Moreover, individual risk factors, including the presence of anticoagulation, coagulopathy, malignancy, recent infections, or any upcoming endoscopies, should be considered when choosing the appropriate approach (as gastroenterologists can perform the block via an ultrasound-guided injection near the posterior gastric wall).
Celiac Plexus Anatomy
The celiac plexus is located on the anterolateral aspect of the aorta bilaterally, surrounding the trunk of the celiac artery. Although it has distinct portions, it forms a complex network of ganglia supplying both parasympathetic and sympathetic innervation as well as receiving visceral afferent nociception from the upper abdominal organs, including the liver, spleen, ascending and transverse colon, stomach, and pancreas. The plexus is typically located at the level of the first lumbar vertebral body (L1). Its ganglia range above and below the L1 level, forming connections with splanchnic nerves superiorly and the superior mesenteric ganglia inferiorly. Since the kidneys are located anterolaterally to the vertebral body at this level, they could be encountered while utilizing traditional retrocrural and anterocrural approaches despite maintaining a medial needle trajectory. Therefore, patients can experience hematuria following the procedure. The inferior vena cava also courses near the right anterior margin of the vertebral body, presenting another potential source of vascular injury in addition to the more readily apparent aorta and celiac artery.
Pros of the Transdiscal Approach to Celiac Plexus Block
The percutaneous transdiscal approach is not dependent on navigating around the vertebral body, thus making advancement more direct and mitigating pain caused by contacting the vertebral body during needle placement. Consequently, it reduces tissue disruption, which could be a source of pain. Since sedation during celiac plexus block has been associated with a lower rate of positive outcomes following neurolysis,2 it is advantageous to utilize the least painful block technique. This has the potential for reducing both false-negative (from procedure-related pain) and false-positive (from the administration of sedation and excessive superficial local anesthetic required to address procedure-related pain) prognostic blocks used to determine suitability for neurolysis. The transdiscal technique also requires less local anesthetic and alcohol or phenol than a bilateral splanchnic nerve block or anterocrural approach, which may further reduce the incidence of a false-positive prognostic block, and decrease the side effects of neurolysis, including inebriation and off-target tissue injury. Because it is a retrocrural approach, spread to the targeted structures is less likely to be impeded by pancreatic tumor burden than with an anterocrural technique. Recent evidence also indicates the transdiscal approach is associated with decreased pain as measured by the visual analogue scale and improved quality-of-life scores compared to the transaortic approach.3
The celiac plexus is located on the anterolateral aspect of the aorta bilaterally, surrounding the trunk of the celiac artery. Although it has distinct portions, it forms a complex network of ganglia supplying both parasympathetic and sympathetic innervation as well as receiving visceral afferent nociception from the upper abdominal organs, including the liver, spleen, ascending and transverse colon, stomach, and pancreas.
The transdiscal method allows for a medial needle trajectory and proximal placement in front of the spinal column, thereby minimizing or eliminating the chance of encountering the kidneys, inferior vena cava, aorta, and pleural cavity and reducing the likelihood of posterior spread of the injectate to spinal nerves. When properly placed just anterior to the center of the T12-L1 disc, it enables increased spread of local anesthetic with lower volumes. Utilizing lower volumes of local anesthetic has been correlated with increased rates of successful neurolysis.2 A single needle can often accomplish the technique if the initial contrast spread is bilateral (Figure 4). By utilizing a single needle, there is also one less tract for a neurolytic agent to spread posteriorly toward the nerve roots or spinal canal.
Cons of the Transdiscal Approach to Celiac Plexus Block
As the technique necessitates going through the intervertebral disc, there is the potential for causing discitis. The risk of discitis is low and likely lower than the risk associated with discography, which is under 0.25% per patient or 0.14% per disc. 4 Due to the avascular nature of both the nucleus pulposus and annulus fibrosus, discitis is difficult to treat and can result in significant morbidity.5,6 The risk can theoretically be reduced by giving preprocedural antibiotics, as well as by utilizing the double-needle technique illustrated in figures 1-5.7 The double-needle technique is hypothesized to reduce the risk of infection by ensuring that the needle that punctures the skin does not also pierce the disc. It reduces the possibility of skin bacteria being translocated into the disc space. The overall benefit of preprocedural antibiotics in preventing discitis is questionable based on systematic reviews.8,9
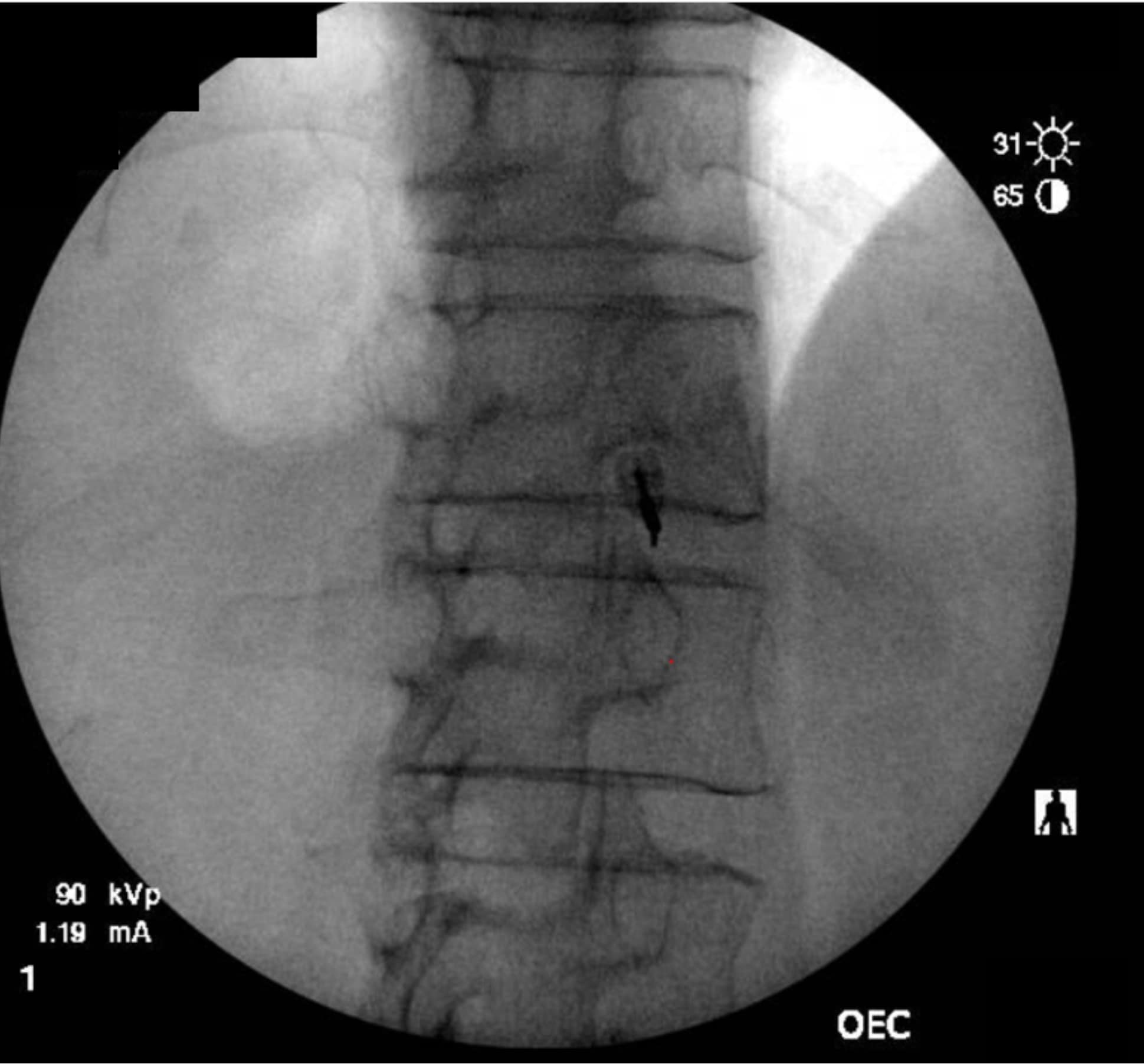
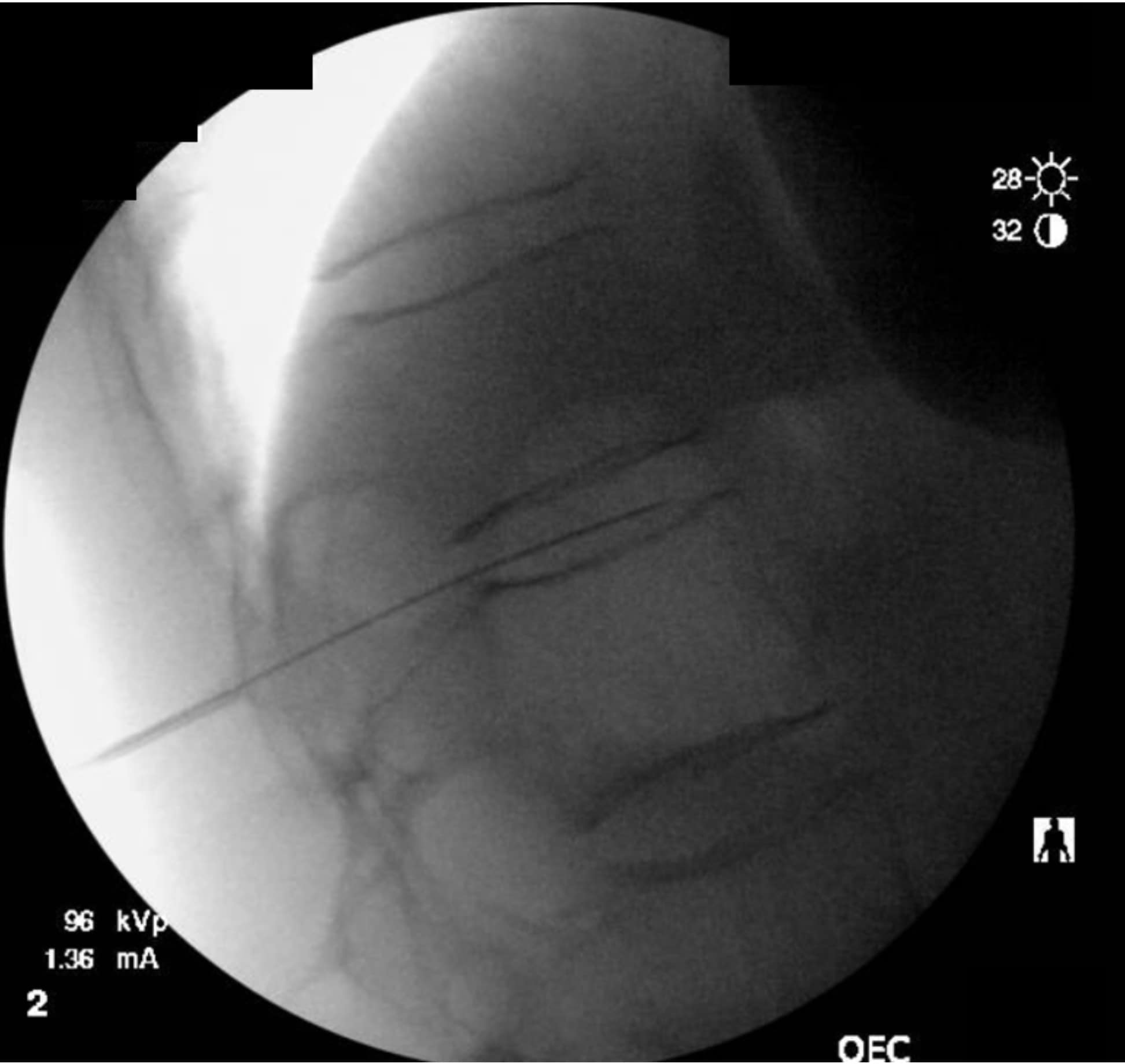
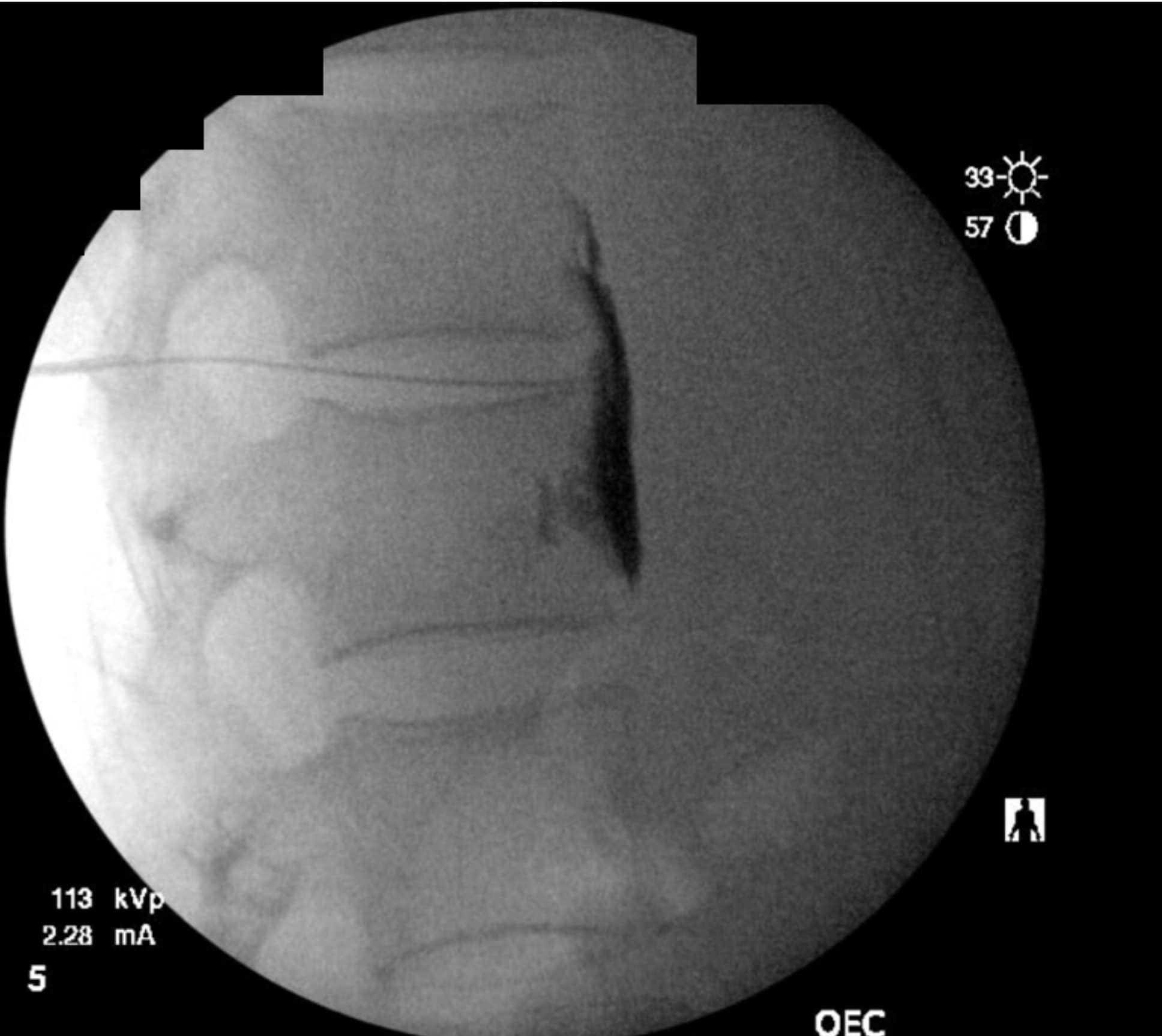
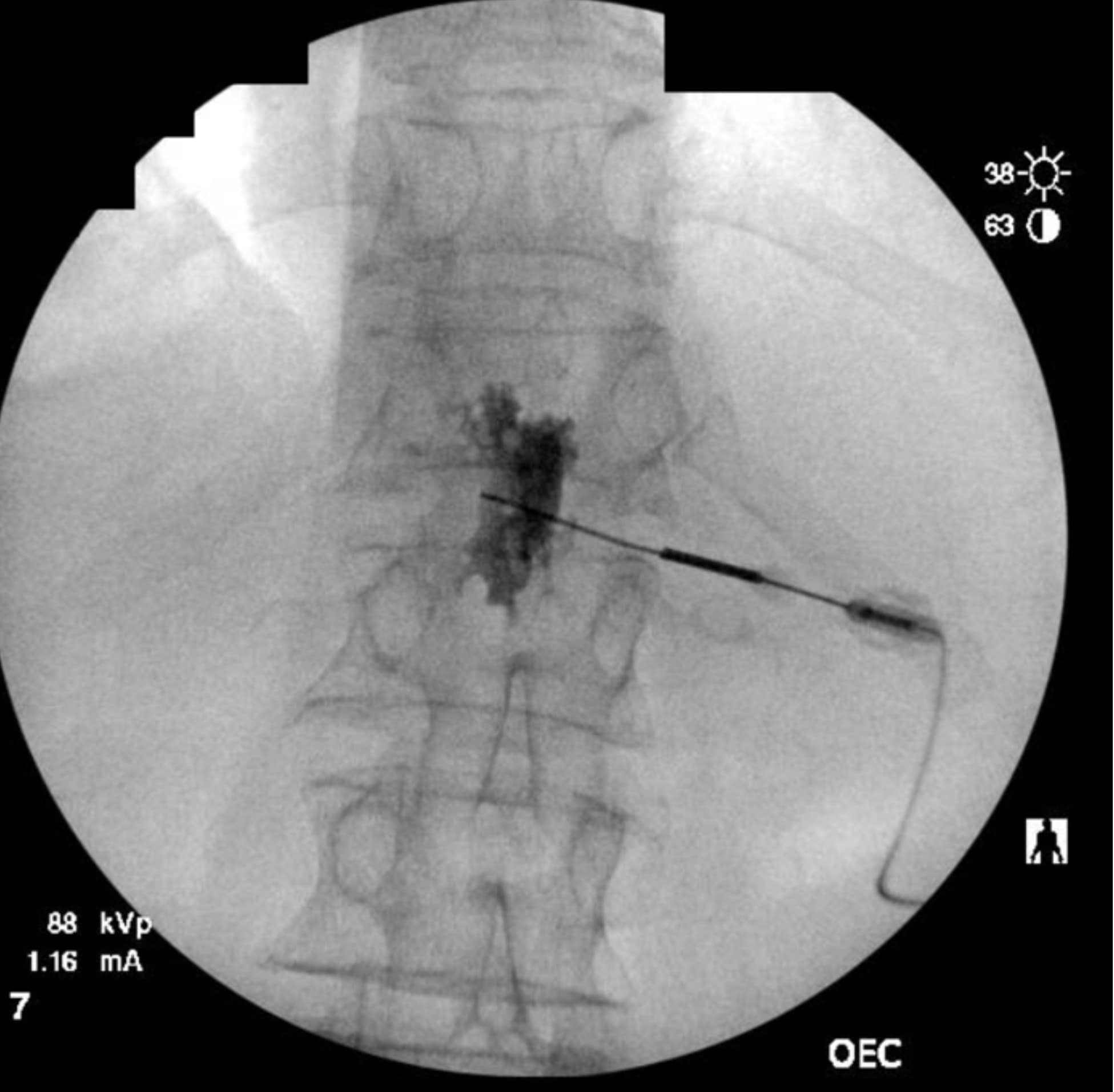
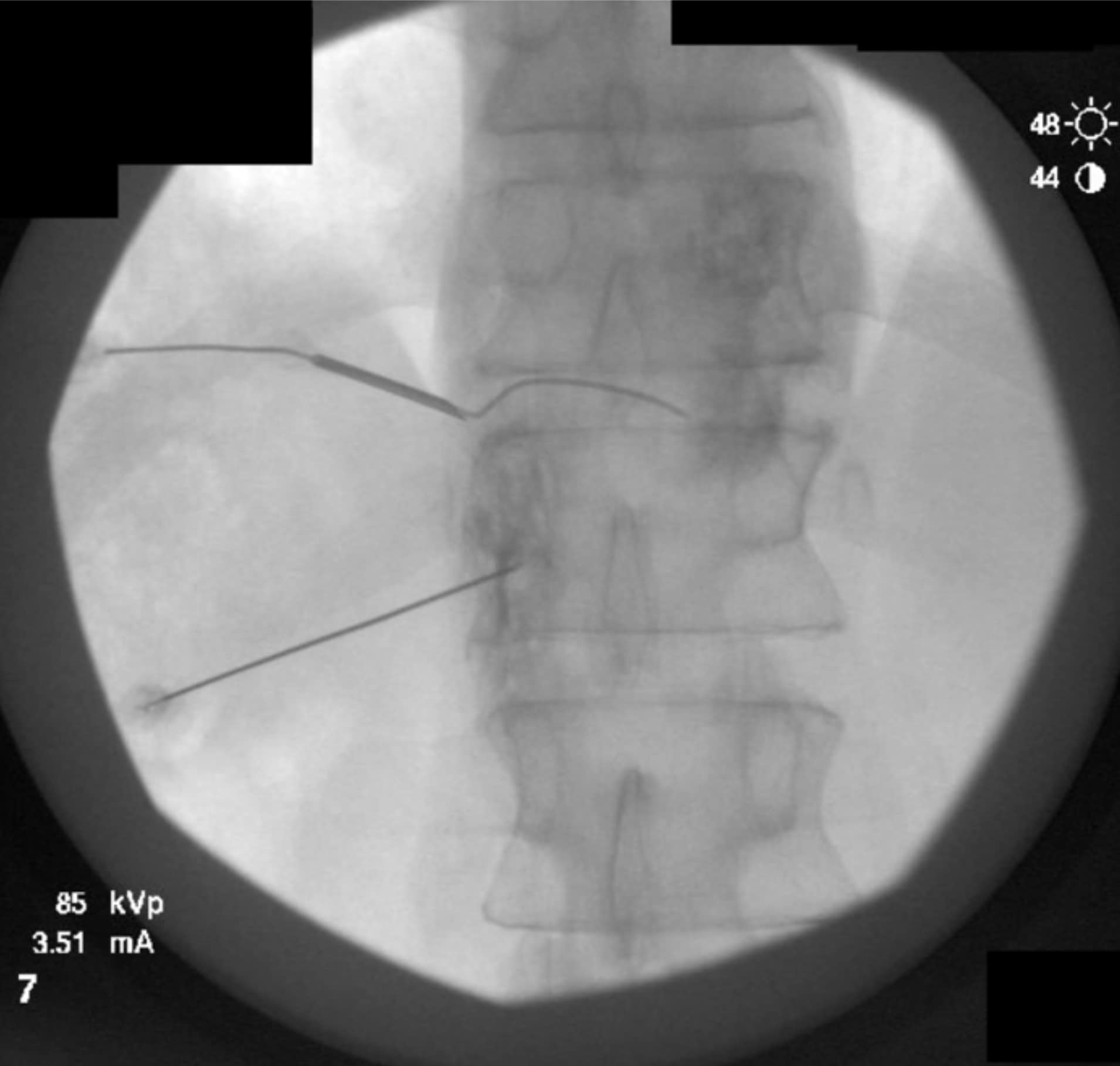
Previous studies in both humans and animals have shown that the sites of disc puncture are predisposed to herniation and accelerated degeneration,10,11 and there is a similar risk with the transdiscal approach. Such concerns are tempered by the fact that changes often take years to progress, and neurolysis of the celiac plexus is typically performed in the setting of a terminal illness in patients who may not be predisposed to disc degeneration (ie, they are not undergoing discography to evaluate a degenerated disc and spinal pain). However, accelerated degeneration might be a pertinent consideration for a patient with back pain being treated for a non-fatal condition or in the setting of a patient whose prognosis is measured in years rather than months, such as with certain types of colon and stomach cancer.
Like other techniques, injury to the celiac trunk or aortic injury is possible. Still, it may be less likely than an anterocrural approach in which the artery is often purposely entered (ie, transaortic approach). To reduce this risk, preprocedural planning after examining the most recent imaging is necessary, and both repeated aspiration and real-time contrast injection are recommended. There is a risk of injectate spread to spinal nerves, but it is less than with a traditional retrocrural approach because the spinal column acts as a natural barrier. Other generic risks of celiac plexus blocks include orthostatic hypotension, diarrhea, spinal cord infarction, and hematoma.
Conclusions
To summarize, the percutaneous transdiscal approach to celiac plexus block is not dependent on navigating around the vertebral body, thus allowing a more direct needle trajectory with less patient discomfort. It may also require less local anesthetic, thereby ensuring fewer false-positive prognostic blocks, and has been associated with improved patient outcomes.3 Moreover, it provides a safer trajectory by avoiding lateral or vascular structures. However, the method may not be appropriate in patients who are expected to live for an extended period as the sites of disc puncture are predisposed to herniation and accelerated degeneration, and there is a remote risk of discitis.7,10,11 Therefore, the technique is most appropriate in patients with a limited life expectancy and challenging anatomy.


References
- Christiansen S and Erdek M. Neurolytic sympathetic blocks. In Benzon HT, Raja SN, Liu SS, et al. Essentials of Pain Medicine. 4th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2018.
- Erdek MA, Halpert DE, Fernández MG et al. Assessment of celiac plexus block and neurolysis outcomes and technique in the management of refractory visceral cancer pain. Pain Med 2010;11(1):92-100. https://doi.org/10.1111/j.1526-4637.2009.00756.x
- Rath A et al. A comparative study of transdiscal versus transaortic celiac plexus neurolytic block. Acta Anaesthesiol Belg 2021;72:121-8.
- Guyer R., Ohnmeiss D, Vaccaro A. Lumbar discography. Spine J 2003;3(3 Suppl):11S-27S.https://doi.org/10.1016/s1529-9430(02)00563-6
- Joyce K., Sakai D, Pandit A. Preclinical models of vertebral osteomyelitis and associated infections: current models and recommendations for study design. JOR Spine 2021;4(2):e1142. https://doi.org/1002/jsp2.1142
- Lacasse M, Derolez S, Bonnet E, et al. 2022 SPILF - Clinical Practice guidelines for the diagnosis and treatment of disco-vertebral infection in adults. Infect Dis Now 2023;53(3):104647. https://doi.org/10.1016/j.idnow.2023.01.007
- Fraser RD, Osti OL, Vernon-Roberts B. Discitis after discography. J Bone Joint Surg Br 1987;69(1):26-35. https://doi.org/10.1302/0301-620X.69B1.3818728
- Willems PC, Jacobs W, Duinkerke ES, et al. Lumbar discography: should we use prophylactic antibiotics? A study of 435 consecutive discograms and a systematic review of the literature. J Spinal Disord Tech2004;17(3):243-7. https://doi.org/1097/00024720-200406000-00013
- Kapoor S, Huff J, Cohen SP. Systematic review of the incidence of discitis after cervical discography. Spine J 2010;10(8):739-45. https://doi.org/10.1016/j.spinee.2009.12.022
- Carragee, EJ et al. 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine 2009;34(21):2338-45. https://doi.org/10.1097/BRS.0b013e3181ab5432
- Sobajima S. et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, x-ray, and histology. Spine 2005;30(1):15-24. https://doi.org/1097/01.brs.0000148048.15348.9b