POCUS for the Pediatric Anesthesiologist
Cite as: Hagen JG, Alrayashi W, Guris RD, et al. POCUS spotlight: POCUS for the pediatric anesthesiologist. ASRA Pain Medicine News 2025;50. https://doi.org/10.52211/asra020125.011.
POCUS Spotlight
Introduction
Point-of-care ultrasound (POCUS) has revolutionized the diagnostic and therapeutic practice of pediatric anesthesiology, providing real-time, critical insights that enhance diagnostic accuracy and procedural safety. As POCUS becomes integral to modern anesthesiology training, established practitioners must incorporate these skills into their clinical repertoire. This review highlights the core POCUS applications for pediatric anesthesiologists, focusing on lung, cardiac, gastric, and vascular assessments, and underscores the importance of continuous skill development to guide and improve patient outcomes (Figure 1).
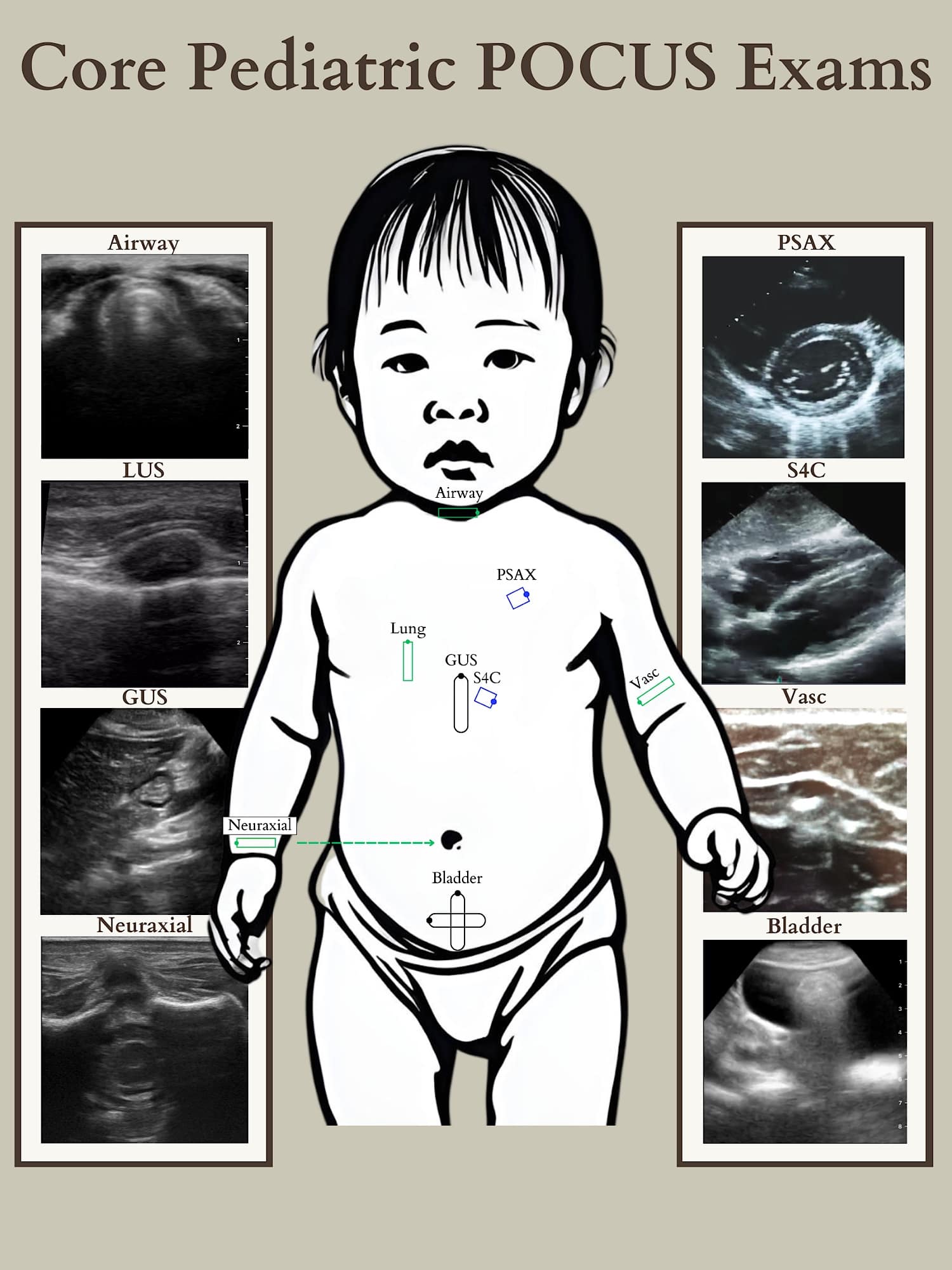
GUS = gastric ultrasound, PSAX = parasternal short axis, S4C = subcostal four chamber, Vasc = Vascular
Reproduced with permission from baby-blocks.com
Lung
Point-of-care lung ultrasound (LUS) is an established imaging modality. To the pediatric anesthesiologist, it is used to assess for pneumothorax, effusion, consolidation, edema, and diaphragm function, is incredibly sensitive at diagnosing endobronchial intubation (which may be desired or undesired depending on the situation), and can facilitate the placement of a chest tube.
The normal lung is an air-filled structure comprised of alveoli, a rich microvascular network, and interstitium. The difference in acoustic impedance between these components generates sonographic artefacts that can be interpreted. In adults and older children, acoustic widows must be found, typically between ribs, through the abdomen, or at the supraclavicular fossa. However, transcostal imaging is feasible in neonates and infants due to their less mineralized, cartilaginous ribs (Figure 2). Comprehensive LUS requires imaging of the entire chest to ensure a complete assessment as a single sonographic image only represents that specific location.
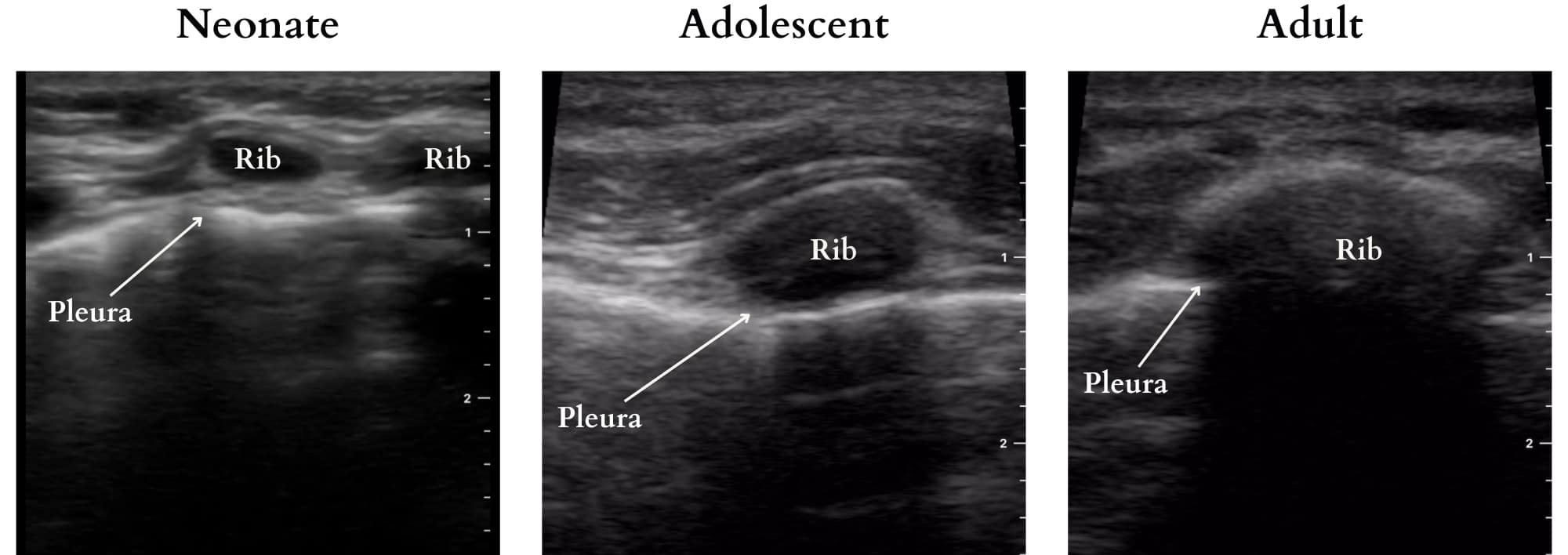
Figure 2. Transcostal imaging highlighting ultrasound penetrability in pediatric patients across age groups.
Reproduced with permission from baby-blocks.com
The basics of LUS are essentially the same across age groups, from neonate to geriatric, and much has been published on image acquisition and interpretation. Familiarization with these fundamentals, the details of which are beyond the scope of this publication, will enable any clinician to perform basic LUS. In this focused review, we highlight two uses that we consider essential skills for every pediatric anesthesiologist. For these assessments, we recommend the use of a high-frequency linear probe. Perhaps counterintuitively, while smaller probes are generally employed for pediatric applications, a larger footprint probe allows for more lung field to be imaged per screen, facilitating a more efficient exam.
Key Views and Findings
Assessment of Ventilation – Lung Sliding
Horizontal sliding of the visceral pleura at the parietal pleura can be seen with respiration. This can be used to confirm the presence or absence of ventilation and is useful when assessing endobronchial intubation. Auscultation has about 60% accuracy in distinguishing between bronchial and tracheal intubation compared to about 90% with LUS.1,2 Notably, lung pulse is seen as small magnitude pleural sliding when heart movement is translated onto adjacent tissue and is a normal feature of the non-ventilated lung.
Pneumothorax, characterized by air between the parietal and visceral pleura, can be identified by the absence of lung sliding and lung pulse. B-lines, also known as comet tail artifacts, are vertical lines originating at the pleura and extending into the lung parenchyma. These lines move synchronously with lung sliding and are caused by reverberation artifacts. Therefore, if any of these are present, there is no pneumothorax at that location. The location at which a ventilated lung should be visible, but lung sliding ceases to be visualized is called a lung point, which represents the separation of the lung from the parietal pleura, and its presence is pathognomonic for pneumothorax. Because the parietal pleura is still present and can generate a reverberation artifact, A-lines can be visible in the presence of pneumothorax. A critical concept in diagnosing pneumothorax via LUS is that conclusions can only be drawn about the imaged area. It is possible to obtain a normal LUS view while a significant pneumothorax exists outside the sonographic field. Therefore, scanning all lung fields before ruling out pneumothorax is imperative.
This review highlights the core POCUS applications for pediatric anesthesiologists, focusing on lung, cardiac, gastric, and vascular assessments, and underscores the importance of continuous skill development to guide and improve patient outcomes.
The sensitivity and specificity of LUS for diagnosing or excluding pneumothorax, particularly in children, is highly variable in the literature.3-6 This variability likely stems from the operator-dependent nature of POCUS and the necessity for a comprehensive evaluation of all lung fields to detect subtle pneumothorax. Given that some studies have shown LUS to be highly sensitive for pneumothorax, addressing educational and technical skill gaps may improve diagnostic consistency rather than abandoning LUS in favor of other imaging modalities.
| Term | Definition |
| A-Lines | Horizontal lines caused by repetitive reverberation artifacts originating at the pleura |
| B-Lines | Vertical lines (also known as comet tail artifacts) originating at the pleura and extending to the bottom of the screen. A small number of these is normal; more than three may represent interstitial pathology. Their presence rules out pneumothorax at that location. |
| Lung Sliding | Horizontal sliding representing the motion of the visceral pleura in the ventilated lung against the parietal pleura. Its presence rules out pneumothorax at that point. |
| Lung Pulse | Short-distance lung sliding in time with the heartbeat seen in areas of non-ventilated lung. Its presence rules out pneumothorax at that location. |
| Lung Point | The point at which lung sliding ceases to be visible in the ventilated lung representing the separation of the visceral pleura from the parietal pleura. Its presence is pathognomonic of pneumothorax, but its absence does not rule out pneumothorax as the lung point may not be visible in large pneumothorax. |
Cardiac
Cardiac POCUS is an invaluable tool for rapidly assessing cardiac structures and function, particularly in perioperative or critical situations, especially when concerns regarding systolic function or significant pericardial effusion arise.7 If any structural abnormality suggestive of congenital heart disease is suspected or visualized while performing an exam, a formal echocardiogram should be performed by a pediatric cardiologist.7,8
Key Views and Techniques
Subcostal Four-Chamber (S4C) View (Figure 3): This view assesses biventricular function, valvular abnormalities, and effusions. To obtain this view, the transducer is placed at the subxiphoid area, centering the indicator at 3 o’clock (toward the left flank) over the heart’s crux, aligning the apex to the right side of the screen. Apply firm pressure, almost flattening the transducer against the abdominal wall, and orient it parallel to the wall, akin to holding a screwdriver.
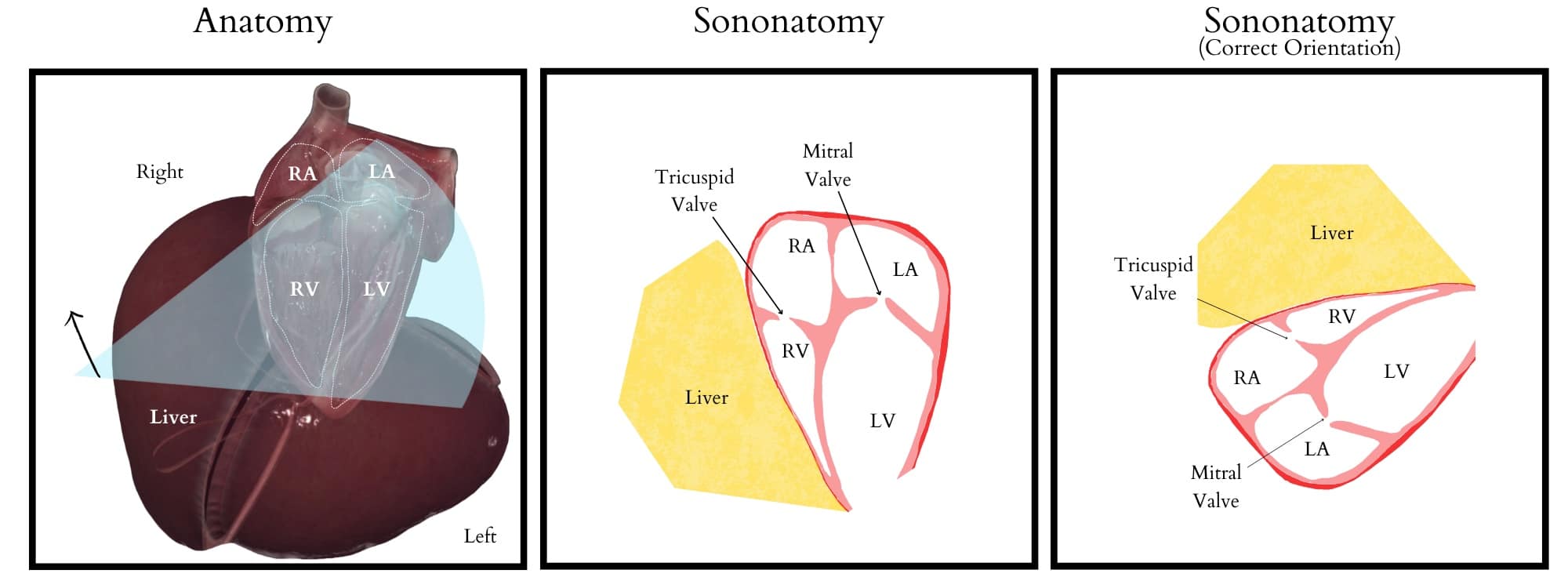
Figure 3. Subcostal four-chamber view illustrating relevant anatomy, sonoanatomy, and ultrasound screen visualization.
RA = right atrium, RV = right ventricle, LA = left atrium, LV = left ventricle
Reproduced with permission from baby-blocks.com
Tips for optimal imaging in the S4C view:
- Slide the probe slightly toward the patient’s right side for improved visualization.
- Position the probe under the xiphoid and angle it upward into the thorax, potentially enhancing the liver view.
- For better imaging, older and more cooperative children can be asked to take a deep breath, which may displace the heart inferiorly, optimizing one’s view
Parasternal Short-Axis (PSAX) View (Figure 4): This view visualizes the heart in cross-section from anterior to posterior. By sweeping the transducer from the right shoulder towards the apex of the heart, transverse cuts at the levels of the aortic valve, mitral valve, and papillary muscles can be obtained. This allows for evaluating left ventricular global and segmental function, right ventricular volume and function, and pulmonary and tricuspid valvular abnormalities. Additionally, this view can be used to assess pericardial effusion. Flattening of the interventricular septum, a recognizable qualitative sign, can indicate increased right ventricular volume (eg, severe tricuspid regurgitation) or pressure overload (eg, pulmonary embolism). Position the ultrasound indicator at 1 to 2 o’clock to achieve this view toward the left shoulder. Center the probe over the left ventricle papillary muscles and the anterior right ventricle, ensuring the left ventricle appears round and centered for optimal visualization.
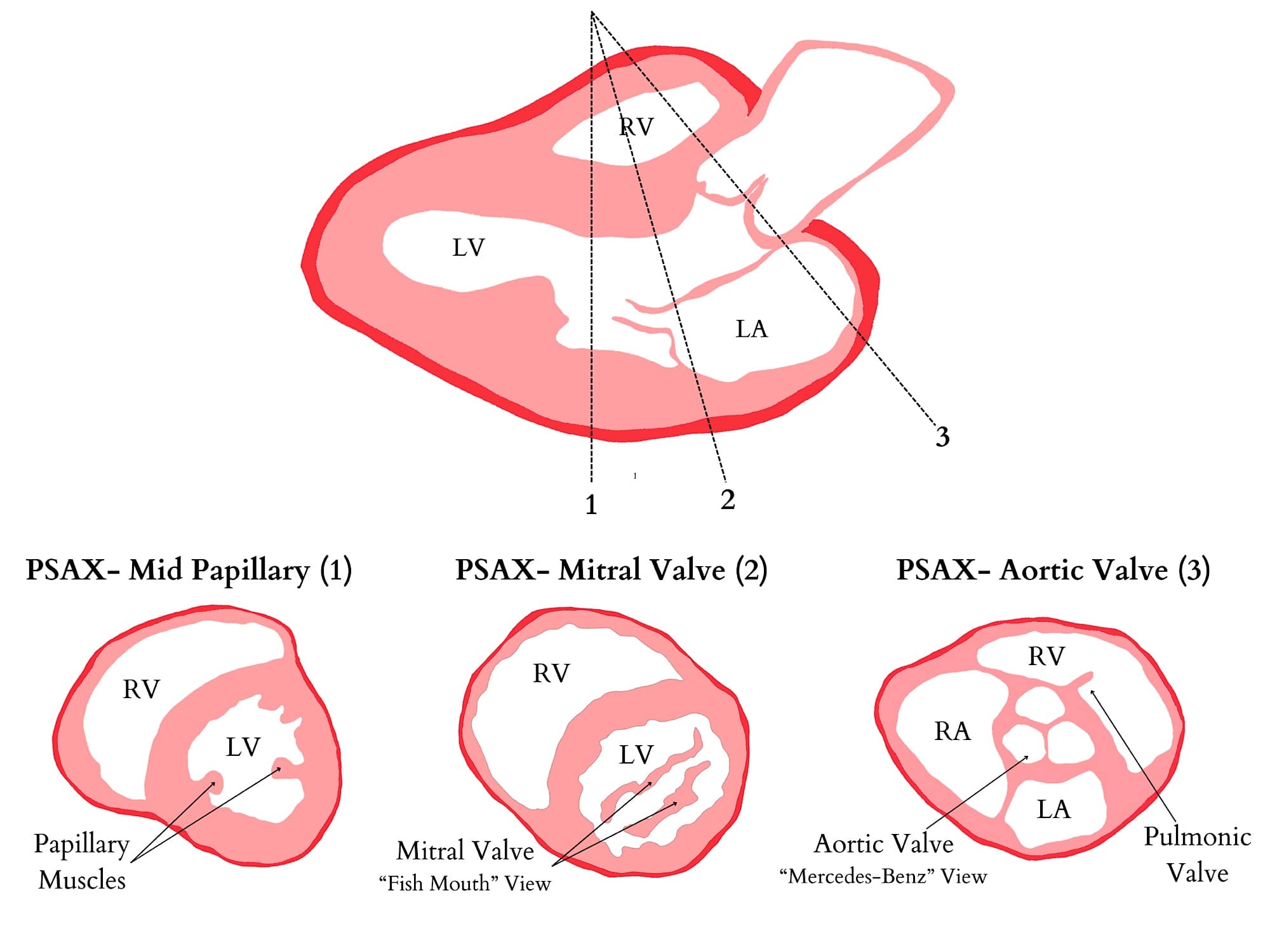
RA = right atrium, RV = right ventricle, LA = left atrium, LV = left ventricle
Reproduced with permission from baby-blocks.com
Tips for optimal imaging in the PSAX view:
- If the left ventricle seems pear-shaped, try scanning one intercostal space higher.
- If the left ventricle appears asymmetric, rotate the probe clockwise or anti-clockwise until it becomes round.
- Advanced tips:
- In a larger child, you might have to scan down as far as the mitral valve, then move down a rib, and finish your sweep.
Gastric
Perioperative gastric ultrasound, a developing field, primarily assesses gastric content before anesthesia to reduce the risk of aspiration. Despite low pulmonary aspiration rates in pediatrics, a thorough evaluation of gastric content may be warranted in specific scenarios for heightened safety and reassurance.9
In pediatric anesthesia, mask induction is a common method for induction in patients under 10. However, a common challenge may arise due to non-adherence to NPO instructions, increasing the risk of inadvertent food intake, especially in patients with communication barriers or developmental delays. With pediatric non-compliance rates to NPO guidelines at 13%, this poses a significant concern10. Furthermore, a clinical adaptation is imperative in many international societies’ ongoing reevaluation and relaxation of NPO guidelines. Recognizing this, a consortium of international experts from various pediatric societies now advocates for gastric ultrasound in children undergoing elective or emergency surgery, offering a proactive approach to ensuring patient safety when NPO adherence is uncertain.11
| Gastric Ultrasound Indications | Description |
| Unknown NPO time | Patients with unclear history and suspicion of non-compliance |
| Comorbidities that delay gastric empty | Bowel obstruction, trauma, acute intraabdominal pathology, diabetes, renal failure, opioid administration, morbid obesity, patients taking GLP- |
| Intraoperative accumulation of gastric content | Assessed during elective ear, nose, and throat surgery in children |
| Diagnosis | Pyloric stenosis, gastroparesis |
| Confirmation of nasogastric tube placement | Used to confirm placement |
Technique
Probe selection: The selection of the ultrasound probe may vary based on patient size and probe availability. Typically, a high-frequency linear probe (12-15MHz) suffices for children under 40 kg, while those over 40 kg may benefit from a low-frequency curvilinear probe (1-5MHz) for better tissue penetration. Handheld devices are also viable options.
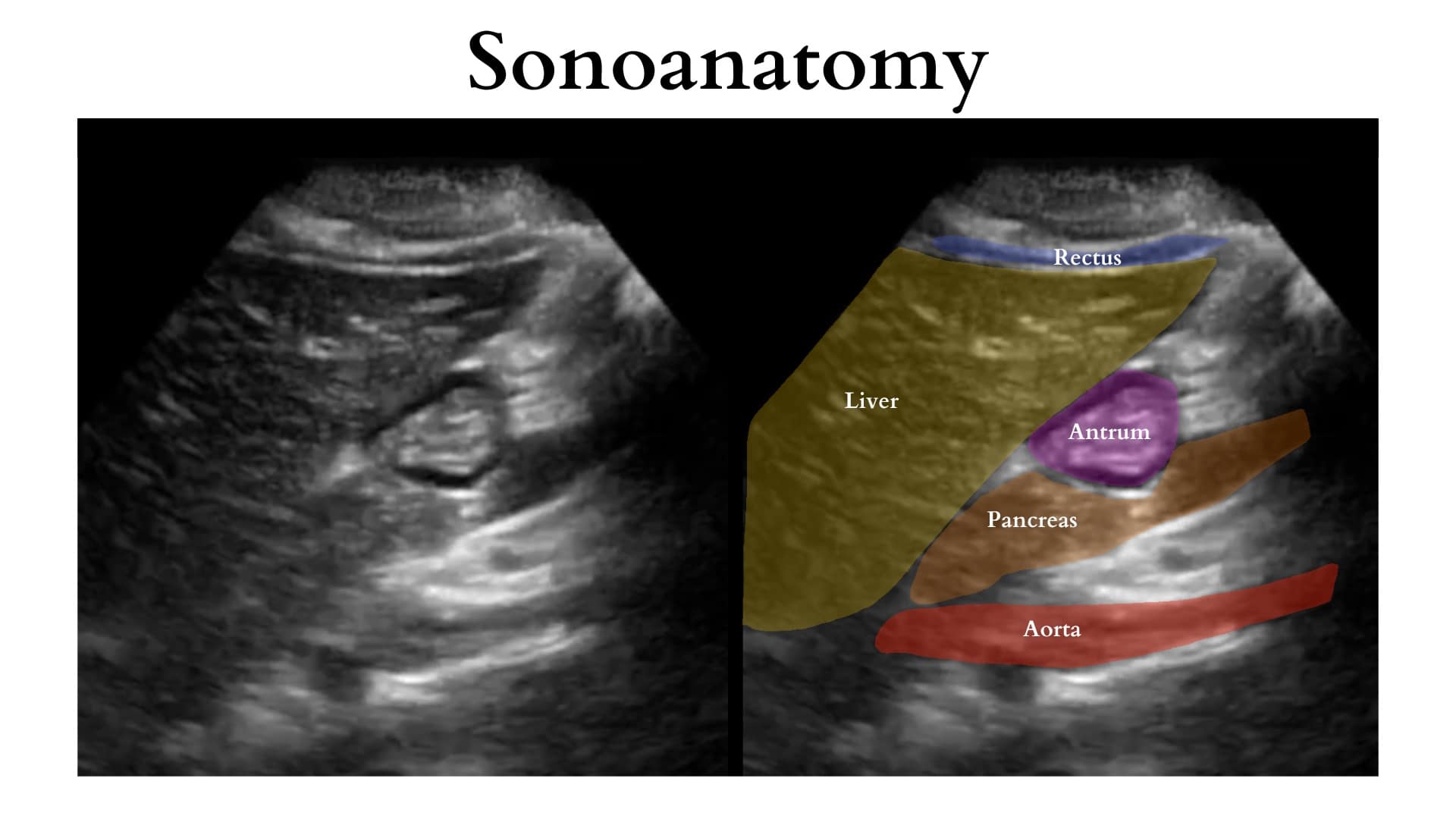
Position and image acquisition (Figure 5): The head of the bed should be raised to 30-45 degrees. Ideally, the patient should undergo scanning in both the supine and the right lateral decubitus (RLD) position. A negative scan in the supine position may not be diagnostic due to stomach content layering. Considering limited patient cooperation in children, starting with a single scan in the RLD position is recommended. First, to identify the antrum during gastric ultrasound, place the probe in the epigastrium and scan it in the sagittal plane. Begin by locating the liver tip and adjusting the probe position by sliding cranially and caudally to visualize it. Next, slide the probe laterally from left to right until the aorta appears in the far field. Typically, the gastric antrum lies posterior to the liver tip and between these two landmarks, the liver tip and the aorta. Once both structures are visualized, the antrum should be identifiable between them. (Figure 6)
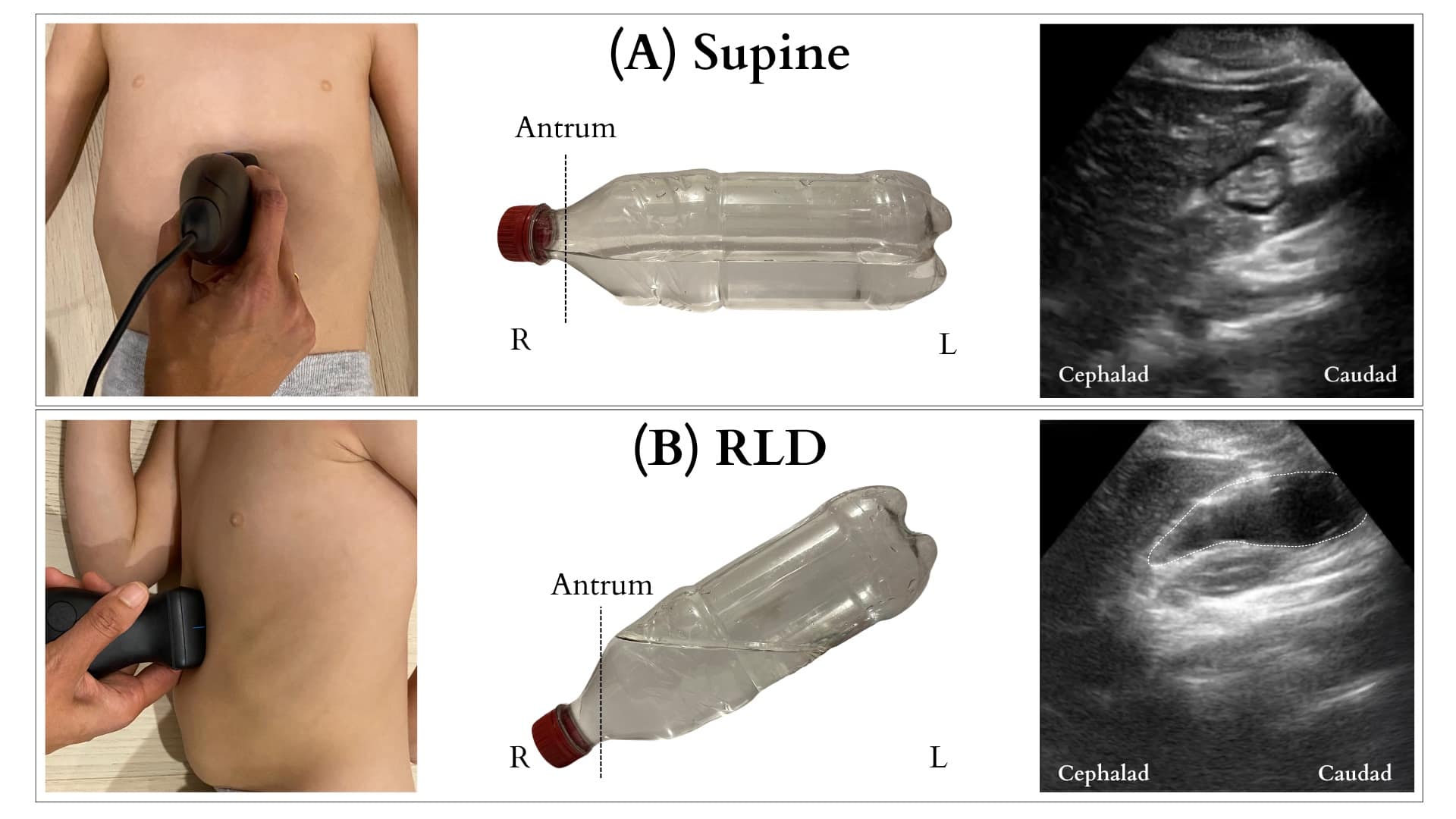
R = right, L = left, RLD = right lateral decubitus
Reproduced with permission from baby-blocks.com
R = right, L = left, RLD = right lateral decubitus
Reproduced with permission from baby-blocks.com
Interpretation (Figure 7): Under ultrasound, the stomach antrum exhibits either empty (Figure 7A) or full states, the latter indicating solid or fluid content. Determining a full stomach with clear fluid involves measuring the cross-sectional area (CSA) of the antrum utilizing the free-tracing method if available or measuring the antral cross-sectional area using the anterior-posterior (AP) and cranial caudal (CC) diameters [CSA = (AP × CC ×π)/4]. (Figure 7B) The CSA is then used to estimate the gastric volume. A volume surpassing 1.5ml/kg is considered above normal. Nonetheless, the threshold for increased aspiration risk remains controversial.12 In contrast, a distended antrum filled with solids exhibits mixed echogenicity with a “frosted-glass” pattern due to the combination of air and solid material post-meal, obscuring the posterior wall and deeper structures (Figure 7C). This distension may persist, with the contents appearing as a compact bolus once most air is expelled (Figure 7D). The presence of solid content confirms a full stomach.
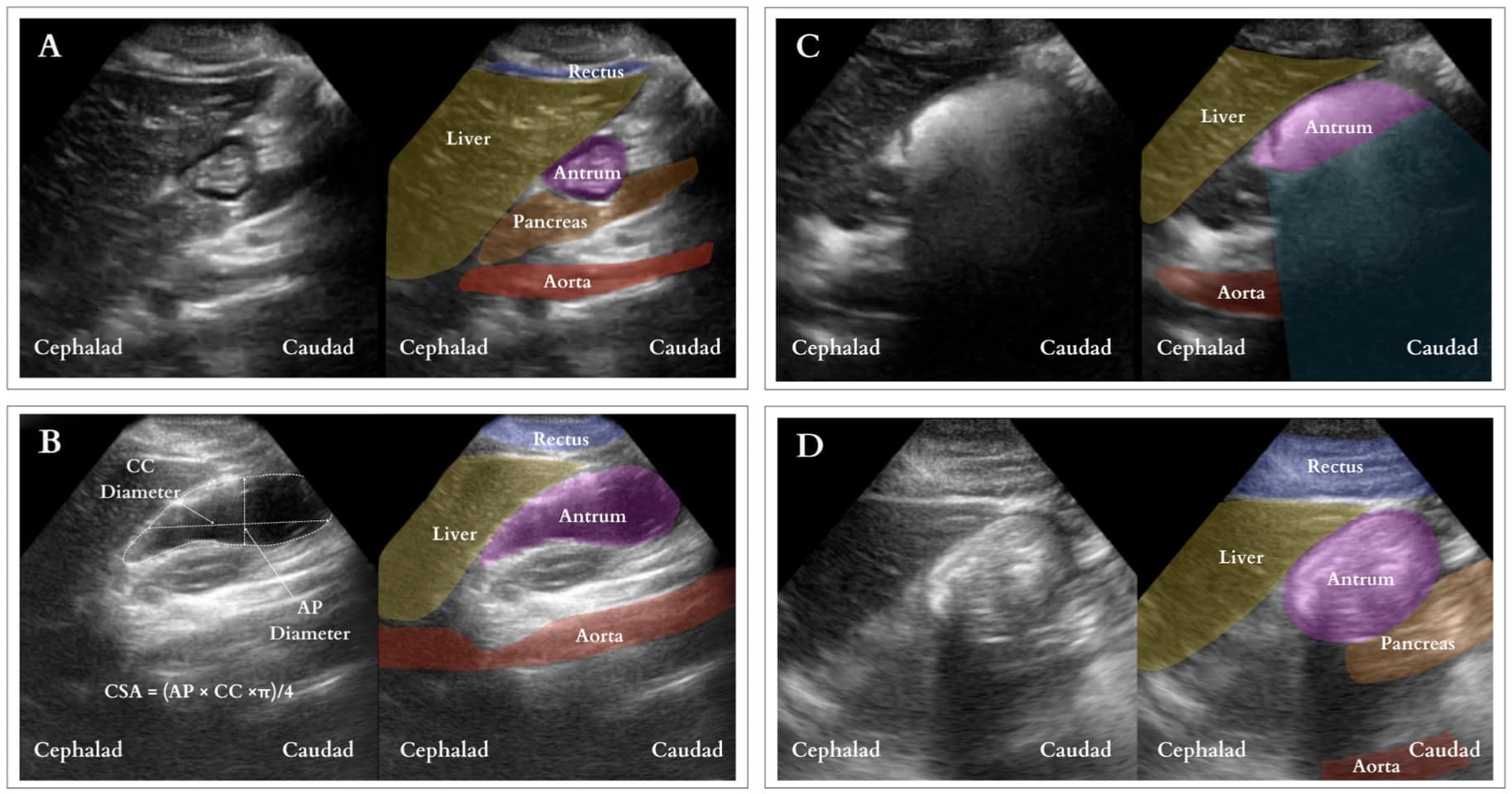
CC = craniocaudal, AP = anteroposterior, CSA = cross-sectional area
Reproduced with permission from baby-blocks.com
Other Indications: Ultrasound can also assist in managing conditions like pyloric stenosis, GERD, and gastroparesis, aiding diagnosis confirmation and treatment guidance. Gagey et al. demonstrated its utility in deciding between RSI and conventional induction for infants with pyloric stenosis after pre-induction suctioning.13 GLP-1 receptor agonists are used for diabetes and weight loss, delay gastric emptying, and raise aspiration risk with studies confirming increased gastric content via ultrasound. The American Society of Anesthesiologists recently provided guidelines for managing patients on GLP-1 agonists, highlighting the significance of ultrasound in assessing aspiration risk, especially when adherence to medication instructions is uncertain.14
Limitations: The primary challenge in utilizing this technique in children is patient cooperation. However, a prospective study conducted on children scheduled for elective surgery demonstrated that ultrasound assessment is feasible in over 95% of children aged 1-16.15 Another potential limitation is the scarcity of literature on pediatric patients, which makes it challenging to conclude ultrasound evaluation of gastric content and its correlation with aspiration risks.
Future trends: As technology advances, emerging trends in gastric ultrasound for pediatric patients include incorporating three-dimensional ultrasound, contrast-enhanced ultrasound, and elastography. These advances have the potential to enhance diagnostic capabilities further, providing a better understanding of pediatric gastric physiology and pathology.
Focused Assessment with Sonography for Trauma (FAST) Exam
An interesting use of POCUS is for the visual confirmation of bladder or gastric tubes. There is minimal data specifically examining the use of ultrasonography for bladder catheter confirmation. However, difficulty with bladder catheter placement still occurs along with several injuries, such as inflating a balloon in the urethra in males.16 Ultrasonography can have the potential to verify or even troubleshoot placement.17 In one study, live transabdominal bladder scanning facilitated a challenging placement in an adult with transrectal pressure.18 Nasogastric tube placement confirmation is presented in the gastric ultrasound section. A Foley catheter is confirmed on a pelvic ultrasound scan. (Figure 8).
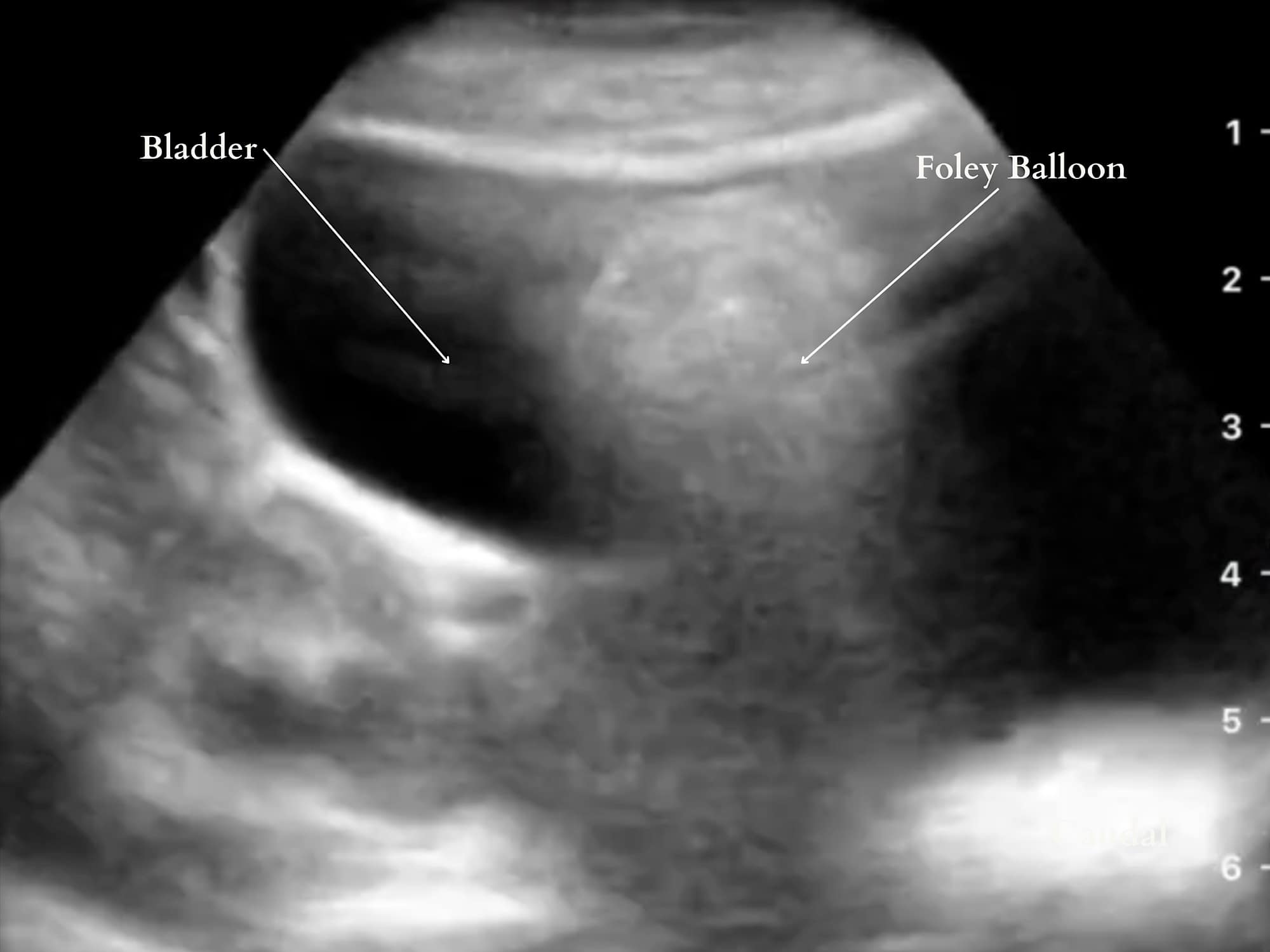
Reproduced with permission from baby-blocks.com
Vascular Access
Obtaining vascular access in pediatric patients, especially infants, can be very challenging. Ultrasound-guided vascular access is especially useful in patients with complex management with multiple venous or arterial sticks that may cause scarring and loss of vascular access. A recent meta-analysis by Heinrichs et al. suggests that ultrasound-guidance may decrease peripheral vascular attempts and procedure time in children in both emergency room and operating room settings.19
Ultrasound is invaluable for obtaining arterial access as it enables dynamic scanning and visualization of the needle tip for optimal placement, thus avoiding the “through and through” method associated with blind arterial sticks. To effectively advance the needle, first move it midline until the tip, appearing as a hyperechoic dot, is visible on the ultrasound screen. Then, keep the needle stationary while moving the ultrasound probe proximally until the needle tip is nearly out of view. Pause the ultrasound probe’s movement and advance the needle until the tip reappears as a hyperechoic dot. Alternate between advancing the ultrasound probe and the needle in small increments, ensuring the needle tip remains visible on the screen throughout the process (Figure 9).
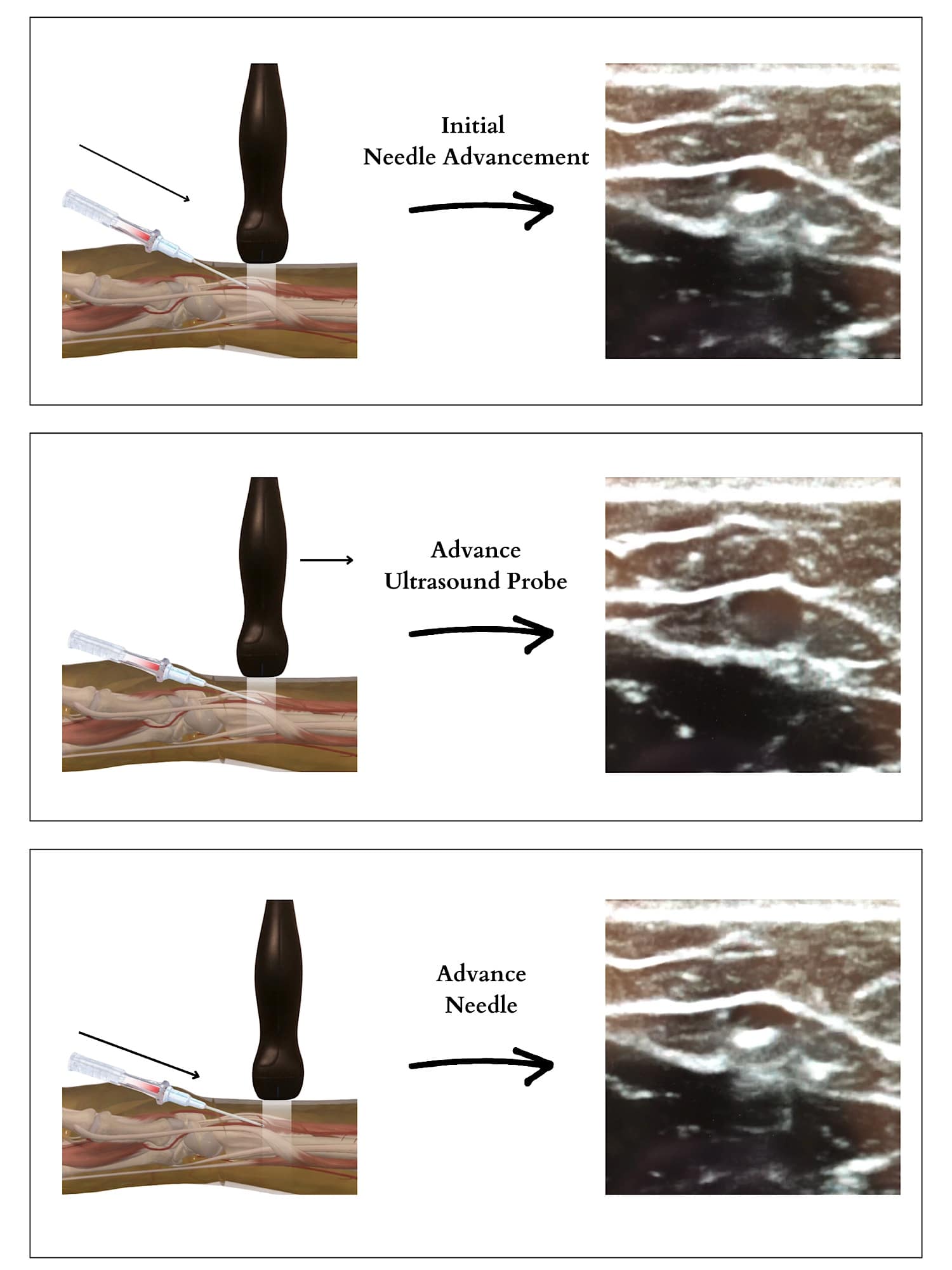
Reproduced with permission from baby-blocks.com
In summary, regardless of whether a clinician opts for traditional blind methods or utilizes ultrasound to facilitate vascular access—be it peripheral, arterial, or central—proficiency in this technique provides an essential tool for addressing challenges in critical locations, such as the operating room, emergency department, or critical care setting.
Airway
Acquiring competency in front-of-neck access in the pediatric patient is a valuable skill set for pediatric anesthesiologists, especially when they are the only specialists immediately available for airway management. In rare and catastrophic can’t-intubate-can’t-oxygenate situations, obtaining access to the airway from the neck is a lifesaving maneuver. The incorporation of ultrasound easily allows for visualization of the cricothyroid membrane (CTM) and marking the exact location for cricothyrotomy (Figure 10).
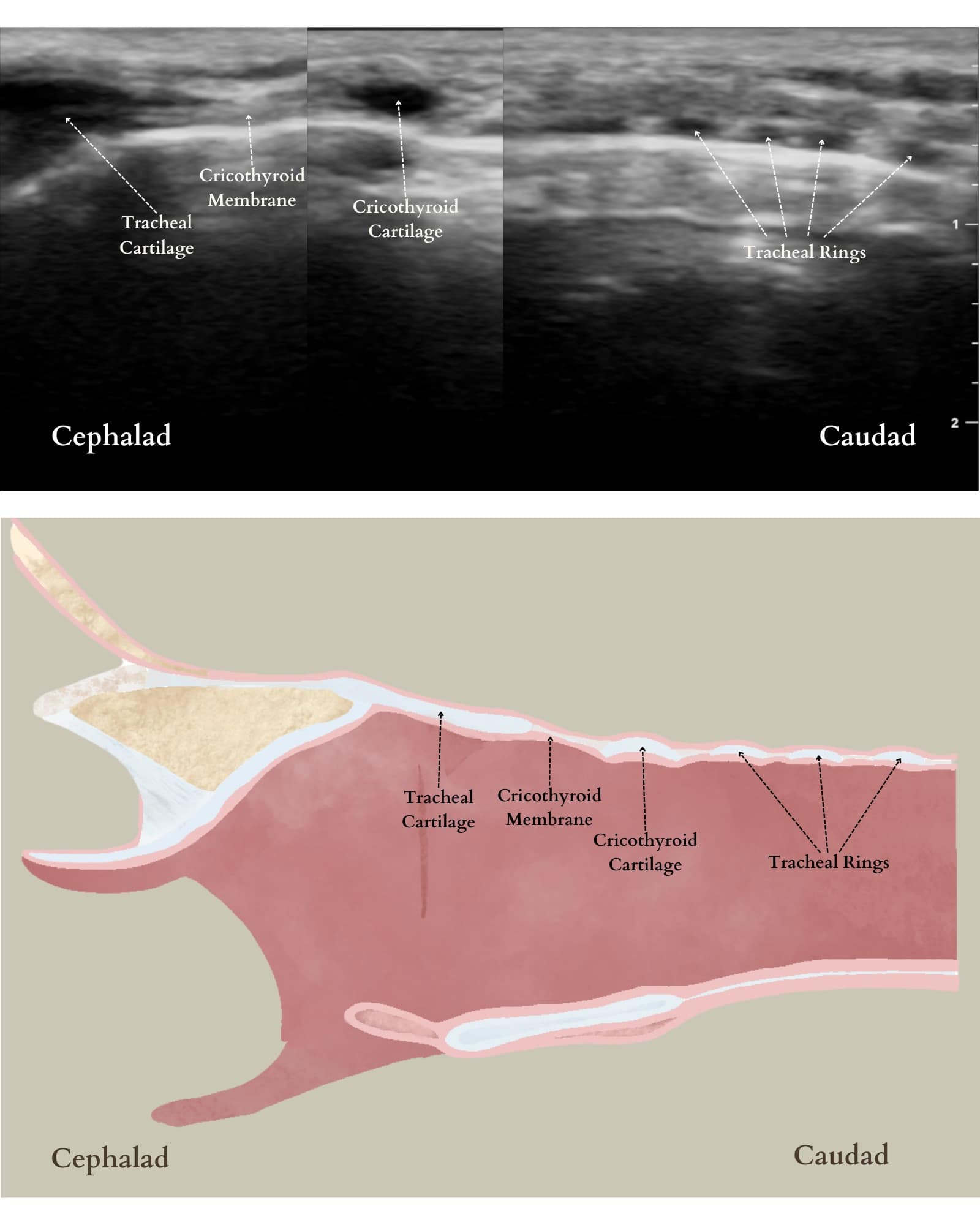
Reproduced with permission from baby-blocks.com
The patient should be supine with the head extended and the ultrasound probe in transverse orientation in the midline of the neck (Figure 11). Once the CTM is located, skin markers can be used to mark the exact location beneath the probe and provide guidance for optimal needle insertion.20
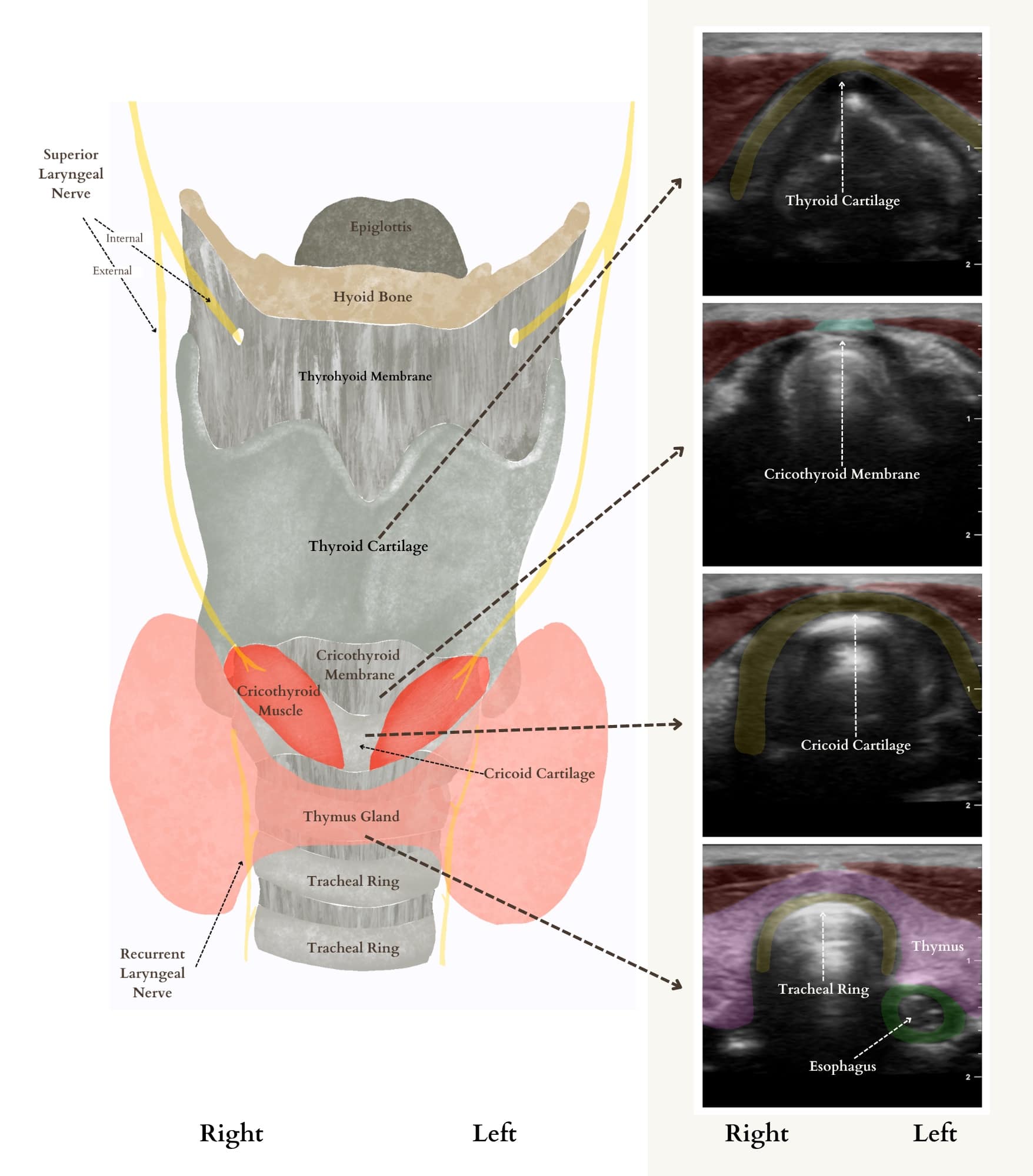
Reproduced with permission from baby-blocks.com
Neuraxial Scanning
The technique for spinal anesthesia placement in infants has not changed for over 130 years.21 The typical approach involves a landmark-based technique using palpation of the vertebral interspaces and blind advancement of the needle into the intrathecal space. However, with the advancements in ultrasound technology, a growing body of literature supports using ultrasound imaging to improve infant success rates. Ultrasound offers excellent views of the infant’s spine due to its lower bone ossification and higher water content (Figure 12). However, the effect of ultrasound on infant LP success rate is unclear and may vary depending on the sonographer’s experience. In contrast, continuous ultrasound guidance rather than the preprocedural ultrasound has been associated with a much higher first-pass success rate. The primary reason is that it has the advantage of needle redirection toward a visible target, limited needle advancement into undesirable structures, and visualization of the injectate at the desired target location in the intrathecal space.21-24
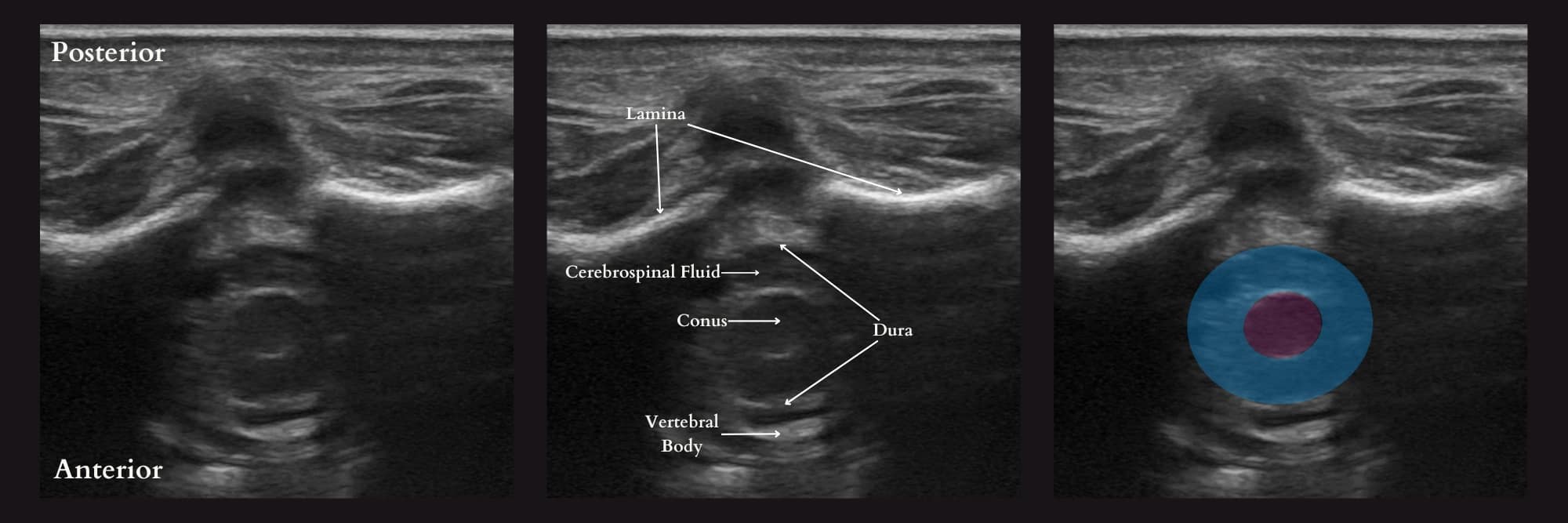
Reproduced with permission from baby-blocks.com
Conclusion
The integration of POCUS in pediatric anesthesiology is rapidly expanding, providing a versatile and efficient tool for bedside clinical assessment and procedural guidance. This article outlines the fundamental POCUS skills of every pediatric anesthesiologist to enhance anesthetic care and related procedures. These core skills encompass essential systems, such as lung, cardiac, airway, and vascular access, providing a comprehensive foundation for integrating POCUS into daily clinical practice. By mastering these techniques, pediatric anesthesiologists can significantly improve patient outcomes and the overall quality of care.




References
- Ahn JH, Kwon E, Lee SY, et al. Ultrasound-guided lung sliding sign to confirm optimal depth of tracheal tube insertion in young children. Br J Anaesth 2019;123(3):309-15. https://doi.org/10.1016/j.bja.2019.03.020
- Ramsingh D, Frank E, Haughton R, et al. Auscultation versus point-of-care ultrasound to determine endotracheal versus bronchial intubation: a diagnostic accuracy study. Anesthesiology 2016;124(5):1012-20. https://doi.org/10.1097/ALN.0000000000001073
- Burton L, Bhargava V, Kong M. Point-of-care ultrasound in the pediatric intensive care unit. Front Pediatr2021;9:830160. https://doi.org/10.3389/fped.2021.830160
- Vasquez DG, Berg GM, Srour SG, et al. Lung ultrasound for detecting pneumothorax in injured children: preliminary experience at a community-based level II pediatric trauma center. Pediatr Radiol 2020;50(3):329-37. https://doi.org/10.1007/s00247-019-04509-y
- Santorelli JE, Chau H, Godat L, et al. Not so FAST—chest ultrasound underdiagnoses traumatic pneumothorax. J Trauma Acute Care Surg 2022;92(1):44-8. https://doi.org/1097/TA.0000000000003429
- DeMasi S, Parker MS, Joyce M, et al. Thoracic point‐of‐care ultrasound is an accurate diagnostic modality for clinically significant traumatic pneumothorax. Acad Emerg Med 2023;30(6):653-61. https://doi.org/10.1111/acem.14663
- Lu JC, Riley A, Conlon T, et al. Recommendations for cardiac point-of-care ultrasound in children: a report from the American Society of Echocardiography. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr2023;36(3):265-77. https://doi.org/10.1016/j.echo.2022.11.010
- Singh Y, Tissot C, Fraga MV, et al. International evidence-based guidelines on point of care ultrasound (POCUS) for critically ill neonates and children issued by the POCUS working group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care 2020;24:1-6. https://doi.org/10.1186/s13054-020-2787-9
- Tan Z, Lee SY. Pulmonary aspiration under GA: a 13‐year audit in a tertiary pediatric unit. Paediatr Anaesth2016;26(5):547-52. https://doi.org/10.1111/pan.12877
- Cantellow S, Lightfoot J, Bould H, et al. Parents’ understanding of and compliance with fasting instruction for pediatric day case surgery. Paediatr Anaesth 2012;22(9):897-900. https://doi.org/1111/j.1460-9592.2012.03903.x
- Frykholm P, Disma N, Andersson H, et al. Pre-operative fasting in children: a guideline from the European Society of Anaesthesiology and Intensive Care. Eur J Anaesthesiol 2022;39(1):4-25. https://doi.org/10.1097/EJA.0000000000001599
- Spencer AO, Walker AM, Yeung AK, et al. Ultrasound assessment of gastric volume in the fasted pediatric patient undergoing upper gastrointestinal endoscopy: development of a predictive model using endoscopically suctioned volumes. Paediatr Anaesth 2015;25(3):301-8. https://doi.org/10.1111/pan.12581
- Gagey AC, de Queiroz Siqueira M, Desgranges FP, et al. Ultrasound assessment of the gastric contents for the guidance of the anaesthetic strategy in infants with hypertrophic pyloric stenosis: a prospective cohort study. Br J Anaesth 2016;116(5):649-54. https://doi.org/1093/bja/aew070
- Joshi GP, Abdelmalak BB, Weigel WA, et al. American Society of Anesthesiologists consensus-based guidance on preoperative management of patients (adults and children) on glucagon-like peptide-1 (GLP-1) receptor agonists. https://www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative. Published June 29, 2023.
- Desgranges FP, Gagey Riegel AC, Aubergy C, et al. Ultrasound assessment of gastric contents in children undergoing elective ear, nose and throat surgery: a prospective cohort study. Anaesthesia 2017;72(11):1351-6. https://doi.org/10.1111/anae.14010
- Kim HH, Tulin-Silver S, Yu RN, et al. Common genitourinary catheters: a systematic approach for the radiologist. Pediatr Radiol 2018;48(8):1155-66. https://doi.org/10.1007/s00247-018-4148-2
- Joseph R, Huber M, Leeson B, et al. Ultrasound-guided placement of a foley catheter using a hydrophilic guide wire. Clin Pract Cases Emerg Med 2018;2(2):143-6. https://doi.org/10.5811/cpcem.2017.12.37045
- Kameda T, Murata Y, Fujita M, et al. Transabdominal ultrasound-guided urethral catheterization with transrectal pressure. J Emerg Med 2014;46(2):215-9. https://doi.org/10.1016/j.jemermed.2013.08.072
- Heinrichs J, Fritze Z, Vandermeer B, et al. Ultrasonographically guided peripheral intravenous cannulation of children and adults: a systematic review and meta-analysis. Ann Emerg Med 2013;61(4):444-54.e1. https://doi.org/10.1016/j.annemergmed.2012.11.014
- Dalesio NM, Kattail D, Ishman SL, et al. Ultrasound use in the pediatric airway: the time has come. A A Case Rep 2014;2(3):23-6. https://doi.org/1097/ACC.0b013e3182a070d2
- Brown TC. History of pediatric regional anesthesia. Paediatr Anaesth 2012;22(1):3-9. https://doi.org/10.1111/j.1460-9592.2011.03636.x
- Tirmizi H. Spinal anesthesia in infants: recent developments. Curr Opin Anaesthesiol 2015;28(3):333-8. https://doi.org/10.1097/ACO.0000000000000199
- Baskin P, Berde C, Saravanan A, et al. Ultrasound-guided spinal anesthesia in infants: a narrative review. Reg Anesth Pain Med 2023;48(12):608-14. https://doi.org/10.1136/rapm-2022-104025
- Alrayashi W, Berde C. Ultrasound-guided “saline myelogram”: confirmation of intrathecal drug delivery despite “dry tap” in infants for spinal anesthesia and spinal therapeutics. Paediatr Anaesth 2022;32(7):883-4. https://doi.org/10.1111/pan.14443