Stellate Ganglion Block for Posttraumatic Stress Disorder: A Call for Clinical Caution and Continued Research
A 53-year-old man, retired United States Naval Officer with more than five combat deployments in support of special operations suffered from posttraumatic stress disorder (PTSD)–like symptoms for 12 years before entering into the study protocol. Initially, medications enabled him to continue on active duty service; however, upon retiring from the military, he noted that his PTSD symptoms became more apparent. Although he had received standard of care mental health visits’ medications and group, family, and individual counseling for PTSD, he ultimately suffered what he called a “nervous breakdown,” resulting in self-medication with alcohol and social isolation from his wife and children. He was formally diagnosed with PTSD in 2010. In 2012 he heard about a study for a rapid treatment for PTSD called the stellate ganglion block (SGB) that could be performed in less than a day. He subsequently sought out the treatment and entered into the study protocol.
He described the procedure as very tolerable, especially in light of his decreased symptoms, which he explained as a sense of wellness and a lifting of his anxiety after treatment No. 1 (placebo). He noted that he felt the best he had in over a decade. He continued to feel good about participating in the study after his subsequent procedure (active SGB treatment) and noted similar, but less dramatic, results as compared to the first procedure. In fact, he felt so good that he was able to go on a trip with his family for several weeks. During the trip he suffered a significant relapse of symptoms that he described as rapid onset over the course of a day. The patient notes that he would gladly undergo such a simple procedure multiple times if he could continue to see the same reduction in symptoms that he had with both injections.
It is estimated that 7 to 8 of 10 Americans will suffer from PTSD. Military populations suffer PTSD at rates estimated at 11–15% since Vietnam, Gulf War, and Operations Iraqi Freedom and Enduring Freedom, and it is estimated to occur at up to rates of 35% if the operational tempo seen in the last decade is maintained.1-2
Yet, current evidence-based PTSD therapies are not without challenges and have limited reach and impact.3 Overall, existing evidence-based treatments have a 30–40% success rate.4-5 However, existing treatment guidelines have often disagreed on first-line therapy. For instance, there is disagreement on the role that pharmacotherapy should play in the treatment of PTSD.
While the Institute of Medicine seemingly downplays the role of medications, the Veteran Affairs/Department of Defense emphasizes the use of medications in their clinical practice guidelines.
Regardless of the treatment guideline chosen, there is a sense that patients have to overcome significant obstacles to receive current evidenced-based treatment options. These obstacles include the stigmata of seeking mental health care, profound pharmacological side effects, and perhaps most insurmountable—the time commitment of weeks, months, and even years necessary for effective therapy.
This has led physicians to explore the potential benefits of alternative therapies for improved clinical management of PTSD in order to find more rapid treatments with longer durations of effect.
SGB case reports indicating immediate, dramatic, and sustained benefit have led to widespread lay press endorsement of the treatments, with reports appearing on Fox News, Time Magazine, and endorsements by Oprah Winfrey. The idea that a one-time SGB could cure PTSD has become so pervasive in society that the authors’ team has been approached by a Congressman and leaders of military units requesting that their patients be flown to the treatment facility in order to receive an SGB.
Although case reports are becoming more common, the block itself has been around for decades and used primarily for indications related to vascular and pain-related conditions. Side effects are rare but can be catastrophic; these include rapid-onset seizures, stroke, respiratory compromise secondary to phrenic and recurrent laryngeal nerve blocks, inadvertent intrathecal and epidural injections, as well as hematoma-induced respiratory insufficiency and local anesthetic systemic toxicity.
The stellate ganglion is a structure in the sympathetic chain commonly found at the level of the 7th cervical vertebra. In 80% of cases, it is a single ganglion formed by fusion of the inferior cervical sympathetic ganglion and the first thoracic sympathetic ganglion; in the remainder of individuals, it is two ganglia in close proximity. By the 1930s, clinicians recognized that injecting local anesthetic into the stellate ganglion, known as a stellate ganglion block, inhibited both efferent sympathetic fibers and visceral pain fibers to the upper extremity and face.6 SGB is now commonly used for the treatment of hypersympathetic activity influencing the upper extremity, such as Raynaud’s phenomenon, or in sympathetically mediated pain as may be present in complex regional pain syndrome.
In 1947, Karnosh and Gardner7 reported a series of cases in which SGBs were used to treat depression. The technique, however, largely was forgotten as a psychiatric treatment until recent cases and popular press reports of SGBs being used to treat PTSD, alcoholism, and menopause.8–14 The mechanism of action of an SGB’s ability to mitigate symptoms in patients with PTSD is unknown. Proposed mechanisms for the SGB’s benefit in patients with a psychiatric condition include downregulation of norepinephrine and/or nerve growth factor. A second theory notes that the SGB procedure should be performed on the right side for patients with PTSD. This proposal is likely because initial case series happened to be performed in patients with right upper extremity pain conditions and PTSD. Correlation with current functional MRI studies has not provided a convincing model to date.
Despite the limited understanding of the mechanism of action of right-sided SGBs to mitigate PTSD symptoms, coupled with the possibility of rare, but catastrophic risks, the appeal for a rapidly acting treatment modality with long duration of action is highly desirable in light of the rising tide of PTSD. Equal to that appeal is the need for further research on the topic to ensure efficacy and safety of SGBs for PTSD. Table 1 summarizes the entire body of published work on SGBs and PTSD at the time the Naval Medical Center San Diego initiated the first randomized controlled trial on this topic.15 Previous published work was entirely composed of case series, totaling 27 patients. Each of these case series had significant methodologic flaws, the most notable being inconsistent follow-up. However, it should be pointed out that a study by Mulvaney et al11 on military populations in 2010 was a turning point in the study of SGBs for PTSD, as it was the first study to use standard outcome measures and to collect data prospectively. Randomized controlled trials and largescale registry data were clearly absent despite widespread clinical use of the procedure.
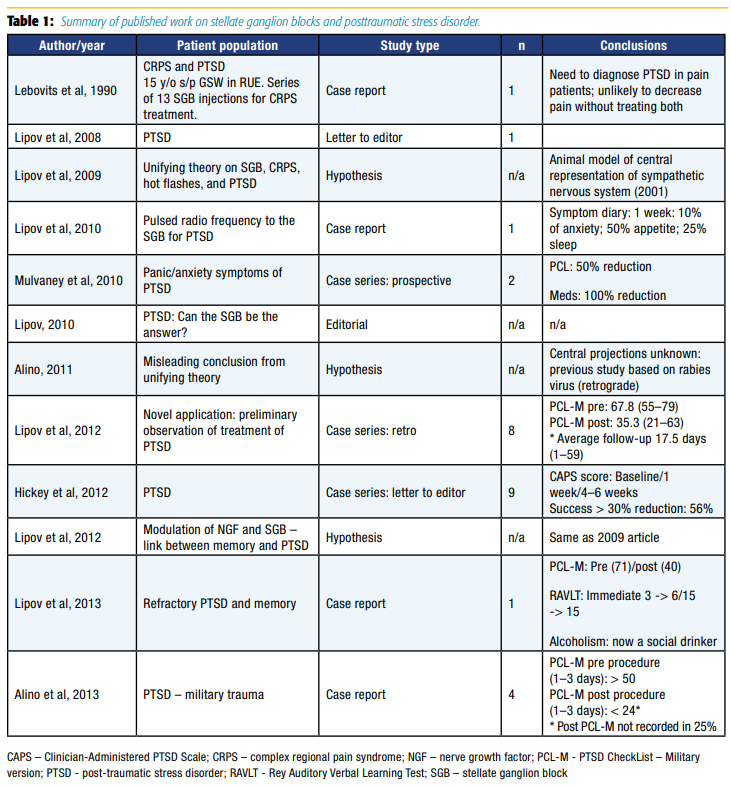
The first randomized, blinded, sham-controlled study to evaluate the efficacy of SGB on PTSD symptoms in a military population was published in Regional Anesthesia and Pain Medicine in 2016.15 In addition to patient-reported symptom severity scores, such as the PTSD Checklist (PCL), this study was the first to require a diagnosis of PTSD by a psychiatrist and used the Clinician-Administered PTSD Scale (CAPS).
Although previous case series have suggested SGBs offer an effective intervention for PTSD, this study did not demonstrate any appreciable difference between SGB and sham treatment. The results indicated that observed PTSD symptoms (CAPS) improved in participants in both the active and sham groups. This was also true for self-reported scores for depression (Patient Health Questionnaire), and anxiety (Beck Anxiety Inventory), but not for self-reported PTSD scores (PCL) or pain (visual analogue scale). The overall magnitude of improvement was modest, less than previously reported in case series. Moreover, improvement with the SGB was not superior to the sham intervention.
The results of this randomized controlled trial differed significantly from a larger retrospective study previously published in the journal Military Medicine in 2014 by Mulvaney et al.16 Mulvaney and colleagues16 observed the response that active duty military patients suffering from combat-related PTSD symptoms had to treatment with SGBs. The authors used a well-validated PTSD symptom severity scale (PCL-Military [PCL-M]) and considered a 10-point change as indicative of a clinically significant improvement. The PCL-M was collected at baseline, 1 week, and each month after treatment up to 6 months. If patients had an initial response and PCL scores after 3 months returned to or were near baseline, they were offered another SGB. In this nonrandomized data set, most patients responded within the first week (79%). This phenomenal response rate seemed to persist at each data collection point (82% at 1–2 months, 74% at 3–6 months). Not only was the response rate significant, but the degree of the response was remarkable with a 22-point average reduction observed.
It is interesting to consider why the results from this study differed so significantly from the randomized trial performed in a similar patient population by experienced physicians with nearly identical technique. Indeed, the differences highlight well the problems with drawing significant conclusions from nonrandomized or retrospective trials or from low-powered randomized controlled trials.
Both articles identified potential bias and possible confounders that could explain the widely disparate outcomes.
In the study of Hanling et al,15 the authors noted that most of their study population had combat-related PTSD. Furthermore, many subjects were in the process of disability evaluation, which in part determines the amount of lifetime disability payments subjects will receive. Both of these factors are associated with a high rate of treatment failures. The fact that all patients showed improvement over time makes this conclusion less plausible, but the possibility still remains given the low number of subjects enrolled in the study.
In the study of Mulvaney et al,16 the authors noted that most of the study population consisted of Special Forces members, highly motivated to redeploy with their units, and that the data were collected retrospectively with low follow-up rates. However, it should be added that without randomization, there is a significant possibility of observer and confirmation bias. For example, the article points out that many patients inflated their PCL scores in order to receive an SGB, once they heard from some of the early participants that the injections helped with symptoms, indicating that patients may have been actively minimizing their symptoms to avoid being stigmatized with a psychological diagnosis and/or not being allowed to deploy with their units. Likewise, patients may have also underreported post-SGB symptoms in order to ensure their return to full duty. A well-powered, randomized, blinded study design with military and civilian populations would mitigate this type of bias, as it would be evident in both the active and control arms of the study.
Ultimately, large-scale, randomized, controlled trials or the formation of an SGB for PTSD registry to track outcomes and determine if any populations in particular receive benefit or harm from this novel treatment of PTSD are needed. Fortunately, it appears that the United States Army Medical Research Acquisition Activity has funded a more definitive multicentered, well-powered study at Womack Army Medical Center, Tripler Army Medical Center, and Landstuhl Regional Medical Center, facilitated by the nonprofit RTI International research organization.
However, until such time as more conclusive studies can be completed, current evidence does not support widespread clinical use of the SGB procedure for PTSD. If it is used, it should be viewed as a bridging therapy meant to minimize PTSD symptomatology to allow increased engagement in existing evidence-based treatment options. In our current clinical practice, we receive requests to perform SGBs routinely on patients with PTSD; therefore, we have established practice guidelines to ensure we maximize the efficacy of these treatments. First, all patients must carry a diagnosis of PTSD confirmed by a mental health professional. Given the limited evidence, we do not perform SGBs for other mental health conditions such as generalized anxiety disorder. Second, we require that our patients have a therapeutic relationship with a mental health professional, as current evidence indicates the SGB procedure to be, at worst, an effective placebo and, at best, a method of symptom management rather than a cure. Third, each patient must complete a baseline biopsychosocial questionnaire that measures relevant parameters related to PTSD as well as follow-up baseline questionnaires every 4–6 weeks to assess the efficacy of the SGB procedures and their overall progress with their condition. Fourth, per previous protocols described in case reports and prospective studies, we perform all SGB procedures under continuous ultrasound guidance on the right side with a standardized dose and volume of local anesthetic (5 mL of 0.25% bupivacaine with 1:400,000 epinephrine). Finally, we perform a series of three SGBs separated by 1–2 week intervals and reassess each patient’s progress via a follow-up visit and follow-up biopsychosocial questionnaire. If the patient is showing substantial progress with PTSD, we continue the SGBs. If the patient has demonstrated minimal or no improvement, we discontinue the SGBs. By following this protocol, we allow patients suffering from PTSD to receive this experimental treatment, while continuously monitoring their progress to ensure optimal outcomes for each patient.
References
- Gradus JL. PTSD: National Center for PTSD. Available at: http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Published February 4, 2017. Accessed February 7, 2017.
- Atkinson MP. Institute for Operations Research and the Management Sciences. A Dynamic Model for Posttraumatic Stress Disorder Among U.S. Troops in Operation Iraqi Freedom. National Meeting: INFORMS Conference; 2009.
- Committee on the Assessment of Ongoing Efforts in the Treatment of Posttraumatic Stress Disorder, Board on the Health of Select Populations, Institute of Medicine. Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Final Assessment. Washington, DC: National Academies Press; 2014.
- Hoge CW. Interventions for war-related posttraumatic stress disorder: meeting veterans where they are. JAMA 2011;306(5):549–551. doi:10.1001/jama.2011.1096.
- Difede J, Olden M, Cukor J. Evidence-based treatment of post-traumatic stress disorder. Annu Rev Med 2014;65(1):319–332. doi:10.1146/annurevmed-051812-145438.
- Theis FV. Effect of sympathetic neurectomy on the collateral arterial circulation of the extremities: experimental study. Surg Gynecol Obstet 1937;57:737.
- Karnosh LJ, Gardner WJ. The effects of bilateral stellate ganglion block on mental depression: report of 3 cases. Cleve Clin Q 1947;14(3):133–138.
- Hicky A, Hanling S, Pevney E, Allen R, McLay RN. Stellate ganglion block for PTSD. Am J Psychiatry 2012;169(7):760–760. doi:10.1176/appi.ajp.2012.111117299.
- Hickey AH, Navaie M, Stedje-Larsen ET, Lipov EG, McLay RN. Stellate ganglion block for the treatment of posttraumatic stress disorder. Psychiatric Ann 2013;43(2):87–91. doi:10.3928/00485713-20130205-08.
- Lipov EG, Navaie M, Brown PR, Hickey AH. Stellate ganglion block improves refractory post-traumaticstress disorder and associated memory dysfunction: a case report and systematic literature review. Mil Med 2013;178(2):e260–e264. doi:10.7205/MILMED-D-12-00290.
- Mulvaney SW, McLean B, de Leeuw J. The use of stellate ganglion block in the treatment of panic/anxiety symptoms with combat-related post-traumatic stress disorder; preliminary results of long-term follow-up: a case series. Pain Pract 2010;10(4):359–365. doi:10.1111/j.1533-2500.2010.00373.x.
- Lipov EG, Navaie M, Stedje-Larsen ET, et al. A novel application of stellate ganglion block: preliminary observations for the treatment of post-traumatic stress disorder. Mil Med 2012;177(2):125–127.
- Lipov E. Successful use of stellate ganglion block and pulsed radiofrequency in the treatment of posttraumatic stress disorder: a case report. Magn Reson Med 2010;2010(580):1–5. doi:10.1002/mrm.1910400110.
- Alino J, Kosatka D, McLean B, Hirsch K. Efficacy of stellate ganglion block in the treatment of anxiety symptoms from combat-related post-traumatic stress disorder: a case series. Mil Med 2013;178(4):e473–e476. doi:10.7205/MILMED-D-12-00386.
- Hanling SR, Hickey A, Lesnik I, et al. Stellate ganglion block for the treatment of posttraumatic stress disorder. Reg Anesth Pain Med 2016;41(4):494–500.doi:10.1097/AAP.0000000000000402.
- Mulvaney SW, Lynch JH, Hickey MJ, et al. Stellate ganglion block used to treat symptoms associated with combat-related post-traumatic stress disorder: a case series of 166 patients. Mil Med 2014;179(10):1133–1140. doi:10.7205/MILMED-D-14-00151.
Leave a commentOrder by
Newest on top Oldest on top