Scrambler Therapy: Thinking Beyond the Gate Control Theory
Cite as: Wang E, Joseph AJ, Smith TJ. Scrambler Therapy: thinking beyond the gate control theory. ASRA Pain Medicine News 2024;49. https://doi.org/10.52211/asra110124.010.
Gate Control Theory as the Prevailing Paradigm
Electrical stimulation has been used since ancient times to treat chronic pain, but modern therapeutic neuromodulation was perhaps inaugurated by Melzack and Wall’s gate control theory in 1965.1 In this theoretical framework, stimulating non-nociceptive Aβ fibers activates inhibitory dorsal horn interneurons, attenuating noxious signals from Aδ and C-fibers.2 Since the first publication describing a spinal cord stimulator (SCS) in 1967, the utilization of SCS and other neuromodulation devices, such as peripheral nerve stimulators (PNS), dorsal root ganglion stimulators (DRG-S), and transcutaneous electrical nerve stimulators (TENS), has steadily grown.3-8 Despite significant differences in indications, technological complexity, and degrees of central versus peripheral mechanisms of action, SCS, PNS, DRG-S, and TENS devices rely in part on the stimulation of Aβ fibers.5,6,9,10 The gate control theory, therefore, continues to underpin the design and function of most contemporary neuromodulation options.
However, implantable neuromodulation devices (ie, SCS, PNS) have an aggregate complication rate (ie, lead migrations, infections, etc.) of 30% to 40%, and despite appropriate trialing and implantation techniques, analgesic failure rates can reach 30%. 11-14 Can a different approach to neuromodulation also provide clinically meaningful analgesia while improving patient safety?
This article briefly overviews Scrambler Therapy (ST), which might hold promise.
ST: Passing Through the Gate (Without Closing It)
ST is a non-invasive method of neuromodulation developed by Giuseppe Marineo at the University of Rome Tor Vergata in the late 1990s.15 ST devices administer low-intensity signals via up to five pairs of cutaneous adhesive electrodes arranged so that each corresponding pair traverses the area of pain. The electrical output comprises 16 unique waveforms combined into 256 distinct sequences, which are dynamically arranged by the software algorithm to avoid repetition and administer a continuously varying pattern.16
Rather than adhering to the gate control theory and stimulating Aβ fibers to inhibit nociception (“closing the gate”), ST is theorized to activate C-fibers and generate action potentials that “scramble” chronic noxious afferent stimuli into sensations perceived as non-painful and endogenous.15 This interruption in the continuous noxious input that patients with chronic pain perceive at baseline potentially mitigates maladaptive central processes.17 Additional studies are necessary to characterize the extent to which ST preferentially stimulates C-fibers. However, the ideal electrical waveform morphology for stimulating Aβ fibers is rectangular (ie, as generated by TENS units), whereas, for C-fibers, it is sinusoidal or half-sinusoidal, such as those produced by ST devices ( Figure 1).18
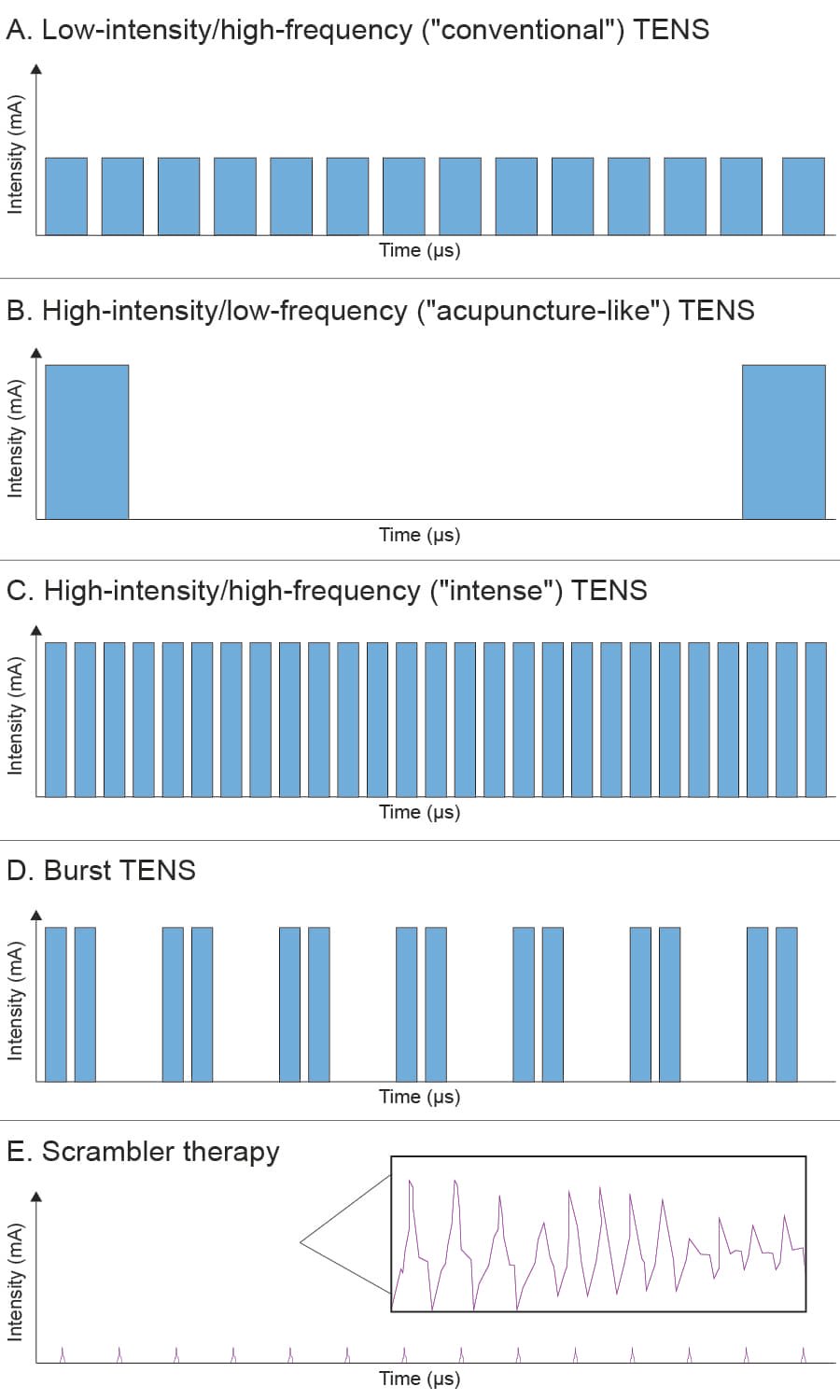
Figure 1. Common waveform patterns in transcutaneous electrical nerve stimulation (TENS) compared to Scrambler Therapy (ST).
Stimulation magnitudes (amplitudes), pulse widths, frequencies, and general waveform morphologies are shown. Panels A, B, C, and D represent prototypical TENS waveforms. Panel E shows a portion of the ST signal, which is grouped into packets of four similar but non-identical waveforms, continuously varied by a proprietary software algorithm. The waveforms in Panels A-E are displayed in proportion and to scale. The inset in Panel E illustrates the morphology of a few representative ST waveforms in greater detail (frequency not to scale).
mA = milliamperes; μs = microseconds
Limited data suggest ST attenuates inflammatory gene expression and improves heat and pressure sensitivity thresholds on quantitative sensory testing.19 ST also induces alterations in cerebral blood flow that are favorable for inhibiting nociception and central sensitization.20 Further studies are necessary to confirm these additional mechanisms of action. Still, these might partially explain the prolonged duration of analgesic effects that have been reported, ranging from weeks to months after a treatment course.16, 21-23
Can a different approach to neuromodulation also provide clinically meaningful analgesia while improving patient safety?
ST is sometimes mistaken for a variation of TENS because both device modalities utilize cutaneous adhesive electrodes. However, the putative mechanisms of action, duration of effect, electrical outputs, and logistics of clinical utilization between these devices are distinct (Table 1). ST is not a subcategory of TENS and is a separate modality.
| Feature | TENS | ST |
| Theoretical Foundation | Gate control theory (stimulation of non-nociceptive Aβ fibers to inhibit nociception from Aδ and C-fibers) | Electrical output resembles action potentials to “scramble” noxious afferent signals into non-painful sensations |
| Primary Nerve Target | Aβ fibers | C-fibers |
| Electrical Output | Rectangular waveform morphology; usually 5-150 milliamperes, 3-9 volts | Sinusoidal or half-sinusoidal waveform morphology; up to 3.5-5.5 milliamperes, 6.5-12.5 volts. Five channels are available; each administers a slightly different signal |
| Most Common Pain Indications | Nociceptive pain (ie, musculoskeletal pain), potentially neuropathic pain | Neuropathic pain, potentially mixed (ie, nociceptive and neuropathic) pain |
| Duration of Analgesia | Effects typically range from hours to days after discontinuation. | Ongoing effects weeks, months, or years after discontinuation have been reported. Some patients require repeat (“booster”) sessions at regular intervals. |
| Restrictions on Use | None; available over the counter, can be self-administered | Restricted to physicians or other healthcare professionals in a healthcare facility |
ST in Clinical Practice
In the United States, ST must be administered in a healthcare facility by a trained operator, although there is no official certification process. ST electrodes are placed proximally and distally to the locations of pain, often along the most closely corresponding dermatomes, while avoiding areas of skin breakage or overt neuropathic symptoms (ie, allodynia, hyperesthesia, etc.) (Figure 2). The ST device is activated at the lowest setting so that the patient perceives non-painful stimuli (sometimes described as “electrical bites”). In 5-10-minute intervals, the operator gradually increases the stimulation magnitude until patients reach their maximal tolerance threshold while not experiencing pain. This process is repeated for 30-50 minutes, comprising one treatment session. Patients generally undergo one treatment session per day for up to 10 consecutive days with the option for fewer sessions if sufficient analgesia has been achieved and maintained. A treatment session is deemed successful if the patient’s symptoms are significantly relieved while the ST device is activated; allodynia and hyperalgesia often diminish within minutes. The initial duration of analgesia might only be for a few hours post-treatment, but it tends to lengthen after each successful session. There is not yet a validated means to predict a specific eventual duration of effect.
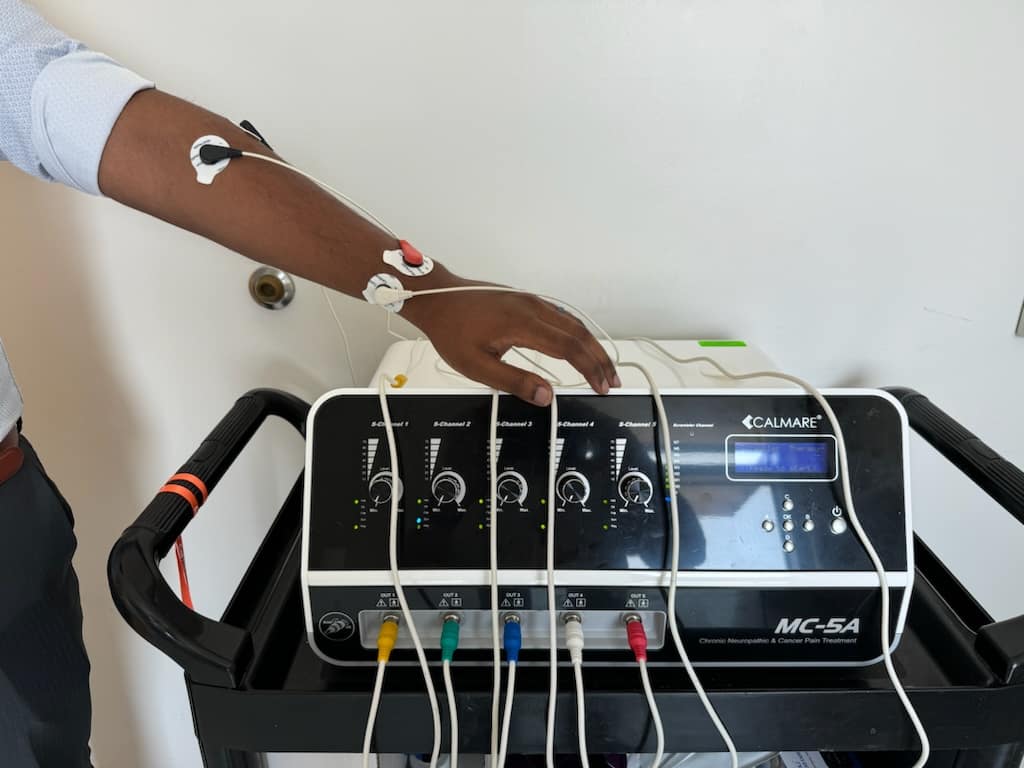
Figure 2. Scrambler therapy machine connected from the left forearm to the left wrist for complex regional pain syndrome.
The electrode pairs for this individual are placed along the C6, C7, and C8 (C8 not pictured) dermatomes. The distal electrodes are placed approximately 2 centimeters proximal to the level of baseline sensory changes. The proximal electrodes are placed along the identical dermatomes.
From our institutional experience, if patients experience prolonged analgesia (ie, weeks to months) after a set of treatments and identical pain symptoms recur, repeat “booster” treatments usually confer similar magnitudes and durations of effect. However, data pertaining to the likelihood of success from repeat ST treatments is not yet available in the literature.
ST Indications and Safety
ST achieved United States Food & Drug Administration 510(k) clearance in 2015 and is indicated for treating chronic intractable pain.24,25 ST appears particularly beneficial for refractory peripheral (ie, chemotherapy-induced peripheral neuropathy [CIPN], painful diabetic neuropathy), or central (ie, neuromyelitis optica) neuropathic pain as well as cancer-associated pain.22,26-29 Recent systematic reviews also support ST’s usefulness for CIPN and refractory neuropathic pain conditions and cancer-associated pain.30, 31 ST may potentially benefit complex regional pain syndrome and nociceptive or mixed pain conditions, but data are sparse.30,32,33 A meta-analysis assessing ST for a variety of chronic pain conditions demonstrated a standardized mean difference of −0.85 (a large effect) between ST and control groups as well as a reduction in opioid use.34
ST is contraindicated in the presence of cardiac implantable electronic devices, vascular clips, implanted neurostimulators (ie, SCS, even if turned off), or skull plates. Still, most orthopedic implants are acceptable (ie, total joint replacements). 35 ST is also contraindicated in patients with epilepsy, and electrodes should not be placed in proximity to the carotid sinus or the heart, owing to a theoretical risk of inducing arrhythmias.35,36 However, in a systematic review specifically assessing the safety of ST, the only reported adverse events among over 1,100 patients were two incidents of contact dermatitis and one instance of minor ecchymosis, all resolved without intervention. This represented a complication rate of 0.26% and zero serious adverse events.37
Ongoing Questions
Whether ST primarily stimulates C-fibers and how this attenuates physiological processes that propagate chronic pain (ie, inflammatory biomarkers and dorsal horn activity) requires further clarification. Also, due to the necessity for continual feedback between the patient and operator for optimal lead placement and stimulation settings, most studies have not blinded their participants, allowing the possibility for reporting biases with notable exceptions.19,20,22
Nonetheless, ST holds promise for many “ideal” pain intervention characteristics.38 It can provide a rapid onset of analgesia, potentially confers a prolonged effect, and has no serious adverse events reported to date. ST warrants further investigation, both for its unresolved questions and its potential.



References
- Francis J, Dingley J. Electroanaesthesia–from torpedo fish to TENS. Anaesthesia 2015;70(1):93–103.https://doi.org/10.1111/anae.12887
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150(3699):971–9. https://doi.org/10.1126/science.150.3699.971
- Shealy CN, Taslitz N, Mortimer JT, et al. Electrical inhibition of pain: experimental evaluation. Anesth Analg1967;46(3):299–305.
- Thomson S. Spinal cord stimulation. International Neuromodulation Society. https://www.neuromodulation.com/spinal-cord-stimulation. Published December 2, 2019. Accessed June 11, 2022.
- Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract2018;18(8):1048–67. https://doi.org/10.1111/papr.12692
- Char S, Jin MY, Francio VT, et al. Implantable peripheral nerve stimulation for peripheral neuropathic pain: a systematic review of prospective studies. Biomedicines 2022;10(10): 2606. https://doi.org/10.3390/biomedicines10102606
- Deer T.R., Pope JE. Dorsal root ganglion stimulation approval by the Food and Drug Administration: advice on evolving the process. Expert Rev Neurother 2016;16(10):1123-5. https://doi.org/10.1080/14737175.2016.1206817
- Transcutaneous electrical nerve stimulation (TENS) devices market size, share, trends. Verified Market Reports. https://www.verifiedmarketreports.com/product/transcutaneous-electrical-nerve-stimulation-tens-devices-market. Published February 2024. Accessed July 26, 2024.
- Chapman KB, Yousef TA, Foster A, et al. Mechanisms for the clinical utility of low-frequency stimulation in neuromodulation of the dorsal root ganglion. Neuromodulation 2021;24(4):738–45. https://doi.org/10.1111/ner.13323
- Mokhtari T, Ren Q, Li N, et al. Transcutaneous electrical nerve stimulation in relieving neuropathic pain: basic mechanisms and clinical applications. Curr Pain Headache Rep 2020;24(4):14. https://doi.org/10.1007/s11916-020-0846-1
- Mekhail NA, Mathews M, Nageeb F, et al. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract 2011;11(2):148–53. https://doi.org/10.1111/j.1533-2500.2010.00407.x
- Eldabe S, Buchser E, Duarte RV. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literature. Pain Med 2016;17(2):325–36. https://doi.org/10.1093/pm/pnv025
- Celestin J, Edwards RR, Jamison RN. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: a systematic review and literature synthesis. Pain Med2009;10(4):639–53. https://doi.org/10.1111/j.1526-4637.2009.00632.x
- Patel SK, Gozal YM, Saleh MS, et al. Spinal cord stimulation failure: evaluation of factors underlying hardware explantation. J Neurosurg Spine 2020;32(1):133–8. https://doi.org/10.3171/2019.6.SPINE181099
- Marineo G. Inside the Scrambler Therapy, a non-invasive treatment of chronic neuropathic and cancer pain: from the gate control theory to the active principle of information. Integr Cancer Ther 2019;18:1534735419845143. https://doi.org/10.1177/1534735419845143
- Marineo G, Iorno V, Gandini C, et al. Scrambler Therapy may relieve chronic neuropathic pain more effectively than guideline-based drug management: results of a pilot, randomized, controlled trial. J Pain Symptom Manage2012;43(1):87–95. https://doi.org/10.1016/j.jpainsymman.2011.03.015
- Smith TJ, Wang EJ, Loprinzi CL. Cutaneous electroanalgesia for relief of chronic and neuropathic pain. N Engl J Med 2023;389(2):158–64. https://doi.org/10.1056/NEJMra2110098
- Schneider T, Filip J, Soares S, et al. Optimized electrical stimulation of c-nociceptors in humans based on the chronaxie of porcine c-fibers. J Pain 2023;24(6):957–69. https://doi.org/10.1016/j.jpain.2023.01.009
- Starkweather AR, Coyne P, Lyon DE, et al. Decreased low back pain intensity and differential gene expression following Calmare®: results from a double-blinded randomized sham-controlled study. Res Nurs Health2015;38(1):29–38. https://doi.org/10.1002/nur.21632
- Lee SY, Park CH, Cho YS, et al. Scrambler Therapy for chronic pain after burns and its effect on the cerebral pain network: a prospective, double-blinded, randomized controlled trial. J Clin Med Res 2022;11(15).https://doi.org/10.3390/jcm11154255
- Wang EJ, Berninger LE, Pantelyat AY, et al. Scrambler Therapy for the treatment of multiple system atrophy-Parkinsonian subtype pain: a case report. A A Pract 2022;16(1):e01560.https://doi.org/10.1213/XAA.0000000000001560
- Mealy MA, Kozachik SL, Cook LJ, et al. Scrambler Therapy improves pain in neuromyelitis optica: a randomized controlled trial. Neurology 2020;94(18):e1900–7. https://doi.org/10.1212/WNL.0000000000009370
- D’Amato SJ, Mealy MA, Erdek MA, et al. Scrambler Therapy for the treatment of chronic central pain: a case report. A A Pract 2018;10(12):313–5. https://doi.org/10.1213/XAA.0000000000000695
- 510(k) premarket notification. U.S. Food & Drug Administration.https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K142666. Published May 22, 2015. Accessed August 7, 2024.
- Delta International Services, Logistics s.r.l. US grants marketing approval for the new Scrambler Therapy®Technology ST-5A Medical Device for the non-invasive treatment of neuropathic and cancer pain (MDR 745/2017). PR Newswire. https://www.prnewswire.com/news-releases/us--grants-marketing-approval-for-the-new-scrambler-therapy-technology-st-5a-medical-device-for-the-non-invasive-treatment-of-neuropathic-and-cancer-pain-mdr-7452017-301229199.html. Published February 17, 2021. Accessed August 7, 2024.
- Coyne PJ, Wan W, Dodson P, et al. A trial of Scrambler Therapy in the treatment of cancer pain syndromes and chronic chemotherapy-induced peripheral neuropathy. J Pain Palliat Care Pharmacother 2013;27(4):359–64.https://doi.org/10.3109/15360288.2013.847519
- Pachman DR, Weisbrod BL, Seisler DK, et al. Pilot evaluation of Scrambler Therapy for the treatment of chemotherapy-induced peripheral neuropathy. Support Care Cancer 2015;23(4):943–51. https://doi.org/10.1007/s00520-014-2424-8
- Smith TJ. Successful treatment of diabetic neuropathy with Scrambler Therapy. J Palliat Med 2021;24(3):320–1.https://doi.org/10.1089/jpm.2020.0658
- Kashyap K, Singh V, Mishra S, et al. The efficacy of Scrambler Therapy for the management of head, neck and thoracic cancer pain: a randomized controlled trial. Pain Physician 2020;23(5):495–506.
- Karri J, Marathe A, Smith TJ, et al. The use of Scrambler Therapy in treating chronic pain syndromes: a systematic review. neuromodulation. 2023;26(8):1499–509. https://doi.org/10.1016/j.neurom.2022.04.045
- Kashyap K, Bhatnagar S. Evidence for the efficacy of Scrambler Therapy for cancer pain: a systematic review. Pain Physician 2020;23(4):349–64.
- Raucci U, Tomasello C, Marri M, et al. Scrambler Therapy® MC-5A for complex regional pain syndrome: case reports. Pain Pract 2016;16(7):E103–9. https://doi.org/10.1111/papr.12474
- Wang AT, Wang EJ, Smith TJ, et al. Scrambler Therapy for patients with complex regional pain syndrome: a case series. J Palliat Med 2023;26(9):1302–6. https://doi.org/10.1089/jpm.2022.0539
- Jin Y, Kim D, Hur J, et al. Efficacy of Scrambler Therapy for management of chronic pain: a meta-analysis of randomized controlled trials. Pain Physician 2022;25(7):E931–9.
- CALMARE™ Model MC-5A non-invasive pain therapy treatment user’s manual. Calmare. http://calmarett.com/media/pdf/User%20Manual_International%20Version_25Nov2008.pdf. Published November 25, 2008. Accessed August 10, 2024.
- Kang R. K201458 Scrambler Therapy technology (model ST-5A) section 510(k) premarket notification. U.S. Food & Drug Administration. https://www.accessdata.fda.gov/cdrh_docs/pdf20/K201458.pdf. Published December 23, 2020. Accessed Month Day, Year.
- Wang EJ, Limerick G, D’Souza RS, et al. Safety of Scrambler Therapy: a systematic review of complications and adverse effects. Pain Med 2023;24(3):325–40. https://doi.org/10.1093/pm/pnac137
- In search of an ideal analgesic for common acute pain. Acute Pain 2009;11(3-4):129–37.https://doi.org/10.1016/j.