How I Do It
Learn from your colleagues. ASRA Pain Medicine members share how they perform common procedures and accomplish other tasks.
- Listed by publication date, most recent on top
How I Do It: Transversus Thoracic Plane and Pecto-Intercostal Fascial Block
This article originally appeared in the November 2019 issue of ASRA News.
The opioid crisis currently threatening the health of the global population has compelled health care providers to reconsider appropriate analgesic options. Massive efforts have been made to maximize multimodal analgesia and regional anesthetic techniques to reduce pain and the need for opioid analgesics. In turn, ultrasound imaging technology has improved and interest in anatomical study has renewed, proliferating fascial plane blocks to assist in the provision of perioperative analgesia.
Treating surgical chest pain adequately and in a manner that minimizes the requirement for significant opioid administration is important.
Although the notion of pain as the fifth vital sign now appears misguided, postsurgical pain limits recovery and may increase the risk of perioperative morbidity. Poorly managed chest wall pain may increase incidence of pulmonary complications because of the need for splinting. Restricted postoperative breathing can result in hypoventilation, atelectasis, pneumonia, and an increased length of hospital stay. Thus, treating surgical chest pain adequately and with minimal opioid administration is important.
Midline chest wall pain can be iatrogenic from median sternotomy and pacemaker placement or pathologic from sternal and medial rib fractures. Chronic pain can develop following poor management of acute pain, contributing to additional physiologic and psychologic stress and consuming disproportionate physical and financial resources.
The transversus thoracic plane block (TTPB), formerly known as the parasternal plane block, is a newer regional technique that provides analgesia to the medial anterior chest wall and may consequently decrease pulmonary morbidity and the need for large-dose opioids. Its indications include:[1]
- Sternotomy
- Sternal fractures
- Medial rib fractures
- Medial coverage for breast surgery
- Medial coverage for placement of a tunneled pacemaker or implantable cardioverter-defibrillator
Sonoanatomy
Muscles
Use a high-frequency linear probe to scan in a parasagittal fashion medial to the midclavicular line over ribs 3 and 4. In this approach, the pectoralis major muscle is appreciated beneath subcutaneous tissue and overlying the ribs. The intercostal muscles lie between the ribs and are superficial to the transversus thoracic muscle (TTM), a hypoechoic band that overlies the pleura. The TTP is found between the internal intercostal muscle and TTM (Figure 1) and is the target for TTPB.
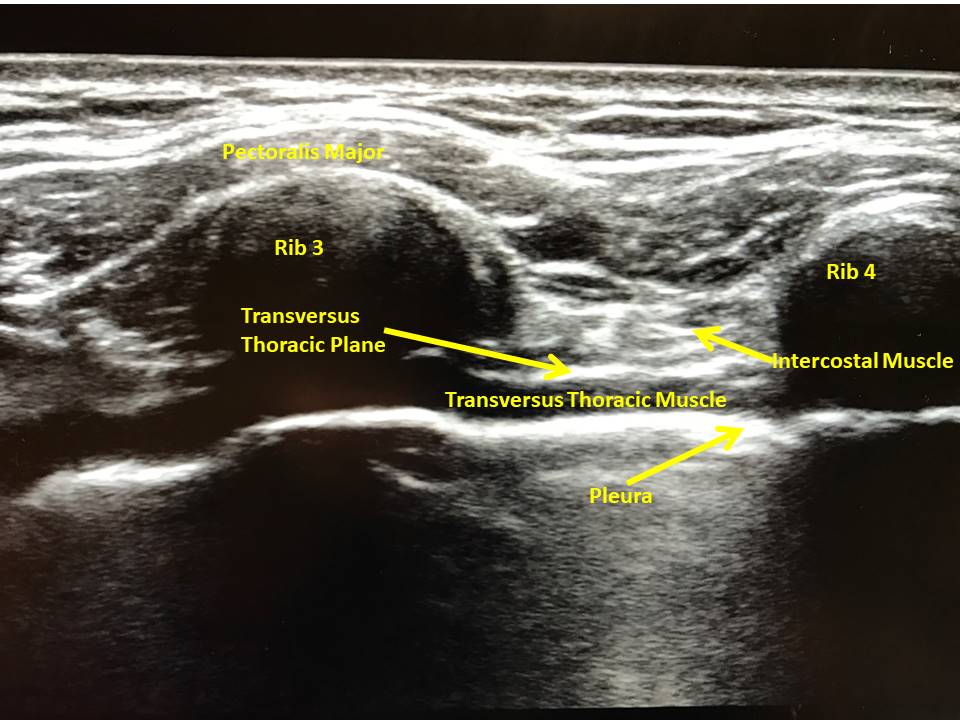
Figure 1:Ultrasound image of transversus thoracic plane with ultrasound held in longitudinal fashion over medial anterior chest wall.
Organs
While scanning the chest wall, the pleura is evident bilaterally as hyperechoic lines with clear lung sliding between the ribs and deep to the intercostal muscle. The pleura should move in coordination with respiratory efforts or ventricular contraction; lack of movement may indicate pneumothorax or other pulmonary pathology. During ultrasound of the left anterior chest wall, the pericardium appears deep to the intercostal muscles. Because of these critical organs’ proximity, take care to visualize the needle tip at all times. If the block cannot be performed safely, use an alternative technique.
Vessels
The internal thoracic artery runs between the internal intercostal muscles and TTM (Figure 2) and should be visualized prior to block placement. In addition to identifying the artery via longitudinal orientation of the ultrasound, placing the ultrasound in the transverse orientation over the plane of interest further confirms the arterial presence so the vasculature can be avoided.[2] Again, constant visualization of the needle tip is vital (Figure 3). Once the TTP has been accessed, aspirate every 3–5 mL to ensure that the needle tip was not placed intravascularly. Although ASRA has neuraxial guidelines for deep plexus and deep peripheral nerve blocks, it has no defined rules for superficial nerve blocks. However, the guidelines advise that vascularity, compressibility, and bleeding consequences be taken into consideration.[3] We therefore recommend evaluating ease of block placement, type of anticoagulation, and vascularity of the site prior to proceeding.
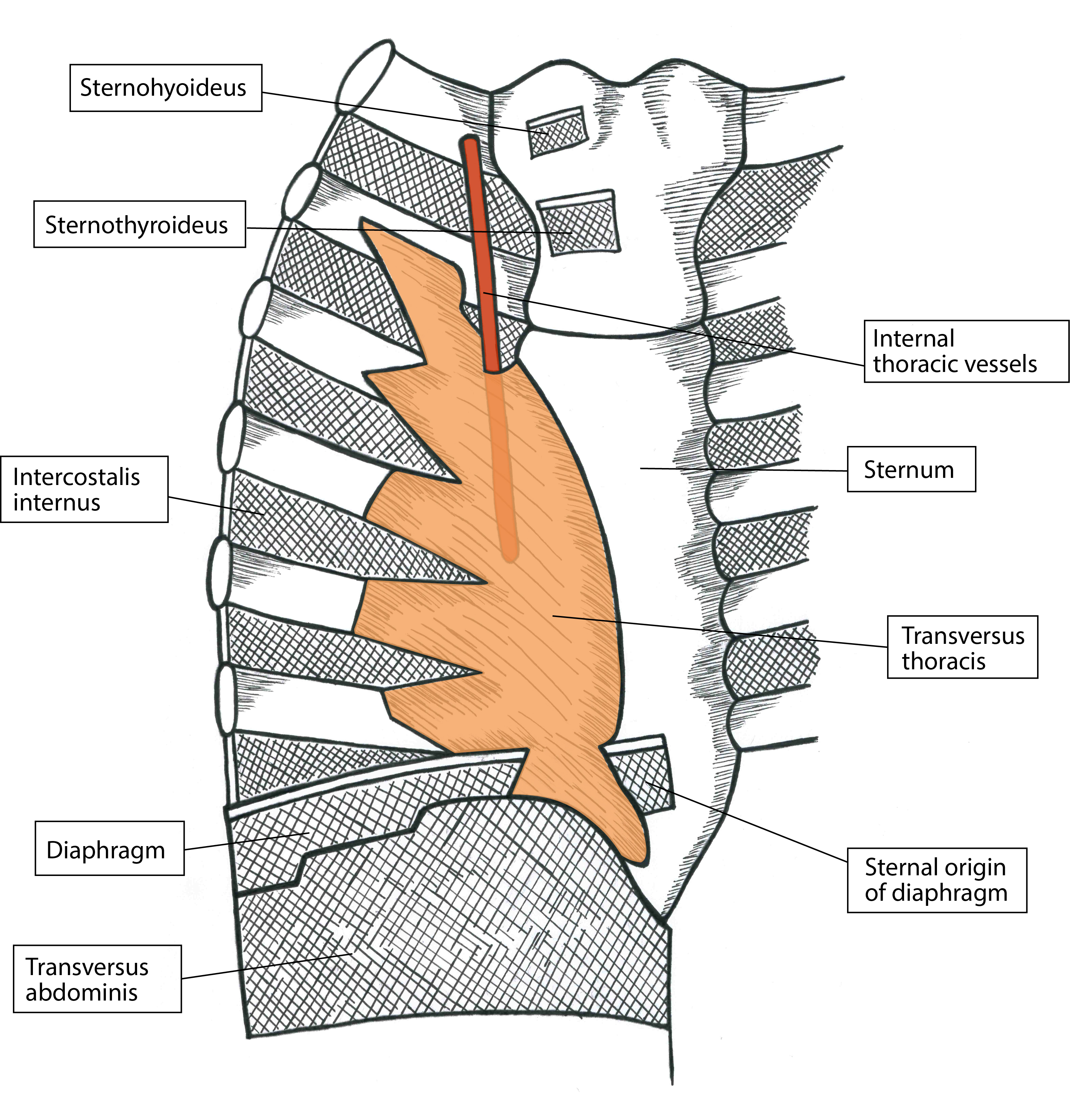
Figure 2: Artistic rendering of anterior chest wall depicting the relationship between the internal thoracic artery and transversus thoracic muscle. Artwork by Athena Ermidis.
Nerves
The sternum’s body is innervated from the anterior cutaneous branches of intercostal nerves 2–6 and the sympathetic plexus around the internal thoracic artery. The nerves lie between the internal intercostal muscle and TTM within the TTP (Figure 4). A collateral branch also aids in supply and runs along the upper border of the rib.[4] The TTPB anesthetizes the nerves via injection of local anesthetic between the two muscles (Figure 3). One injection into this plane on each side of the sternum between ribs 3 and 4 will spread to cover the entire sternum; multiple injections on one side of the sternum are unnecessary if the correct plane is used. Performing the block preoperatively under ultrasound guidance and appreciating no hemodynamic derangements during median sternotomy intraoperatively confirms appropriate block placement.

Figure 3: Ultrasound-guided transversus thoracic plane block.
Osseous Structures
Ideally, ribs 3 and 4 should be visible on the ultrasound screen with an accompanying posterior acoustic shadow. Simultaneously visualize the entirety of the needle and direct it at a steep enough angle to avoid moving beneath a rib where visualization would be obscured (Figure 3). Excessive needle insertion may result in pleural puncture or pneumothorax bilaterally or pericardial injury on the left side of the chest.
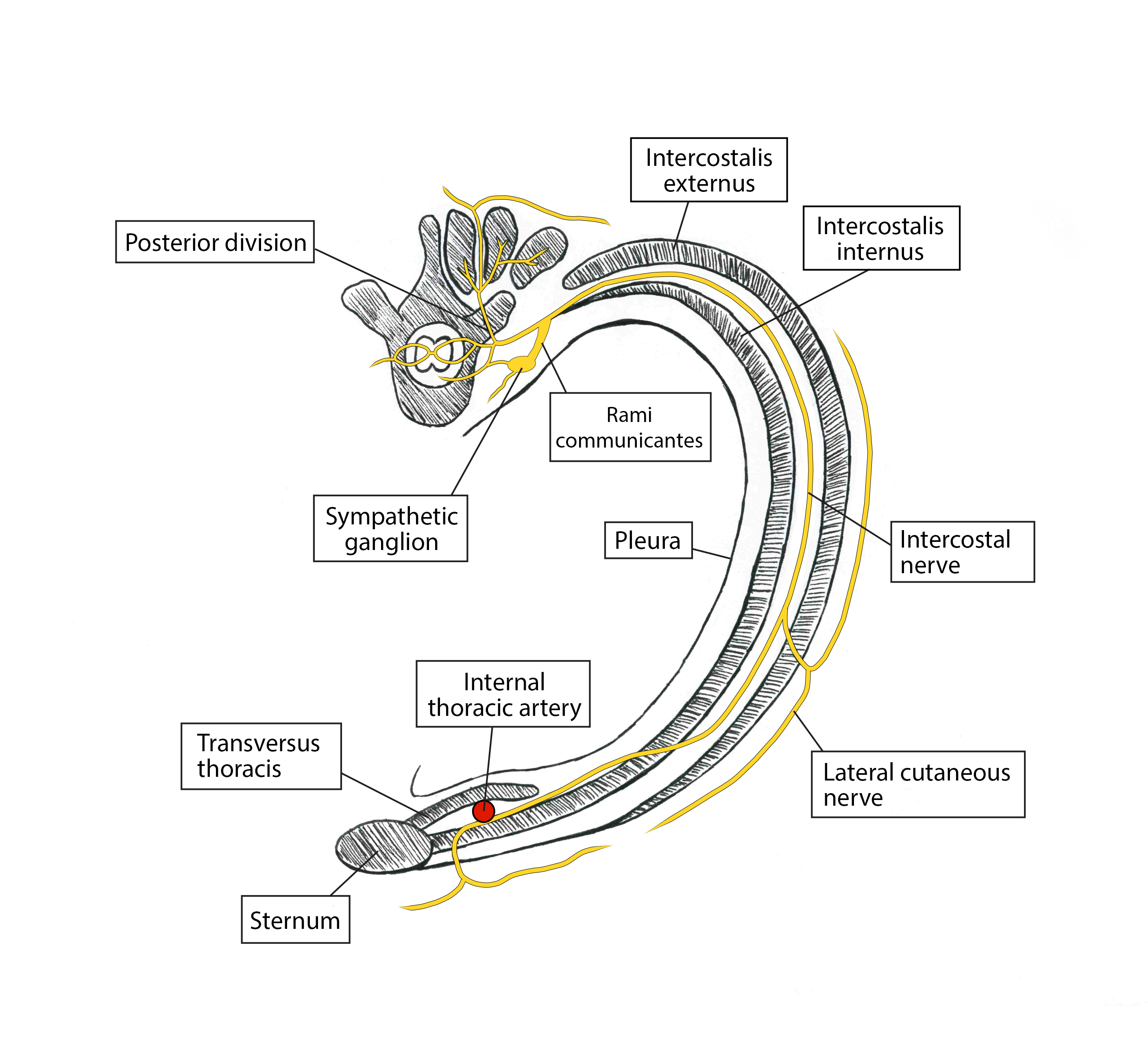
Figure 4: Artistic rendering of transverse view depicting nerve supply of anterior chest wall. Artwork by Athena Ermidis.
Patient Positioning and Equipment Selection
Needle
Use a 21-gauge, 5 or 10 centimeter, blunt tip echogenic needle. Determine the needle length by clinical judgement based on patients’ body habitus and relevant anatomy.
Scanning Technique
Patients can be positioned either supine or with their head up to aid with respiratory status if needed. Place a 50 mm, high-frequency linear ultrasound probe on the chest in the parasagittal plane in the midclavicular line and identify the third or fourth rib space, ribs, pleura, pectoralis major, and intercostal muscles (Figure 1). Scan laterally to medially to visualize the hypoechoic TTM lying deep to the intercostal muscle and superficial to the pleura. The needle target is the plane between the internal intercostal muscle and the TTM (Figure 3). Take care because the internal thoracic artery also runs in the plane; identify it in cross section via an ultrasound scan in the transverse plane.[1] Use color flow doppler over a visible pulsatile structure to confirm presence of the artery; if vascular presence is uncertain, place the probe over an adjacent rib space to evaluate for arterial presence.
Medication Selection
Local Anesthetic
Use bupivacaine 0.25% or ropivacaine 0.25% (10–20 mL on each side). A patient’s weight, type of surgery, location of artery, and risk of vascular puncture should be considered when determining volume and concentration of local anesthetic.
Adjuvants
Preservative-free dexamethasone (1–3 mg on each side) has anti-inflammatory properties and limits ectopic discharge in neural membranes.
Clonidine (0.5 mcg/kg with a max dose of 150 mcg) allows for prolongation of the block via vasoconstriction secondary to hyperpolarization of gated channels. However, data are conflicting on block duration, and it carries a risk of hypotension with higher doses or intravascular injection.
Epinephrine (5–10 mcg/mL) can prolong block duration via vasoconstrictive activity when used with lidocaine and mepivacaine but not ropivacaine. It has neurotoxic potential because of vasoconstriction, and hypertension and tachycardia could alert the regional team to vascular injection.
Buprenorphine can block voltage-gated sodium channels. It has antihyperalgesic properties and can prolong blocks but carries a risk of postoperative nausea and vomiting.
Limited data exist for liposomal bupivacaine, but it has not proved to be more effective or longer lasting than bupivacaine with preservative-free dexamethasone.
Nerve block catheters should be placed postoperatively to avoid surgical field contamination. Adjuvants are not required for block prolongation, but catheter migration and increased risk of infection are concerns.[5]
Description of Technique
The goal is to block the anterior cutaneous branches of intercostal nerves 2–6, which innervate the sternum as originally described by Ueshiema et al.[6] Disinfect the chest and apply sterile ultrasound gel. Place a 50 mm, high-frequency linear ultrasound probe on the chest in a parasagittal plane over the third and fourth ribs at the midclavicular line. Identify the pectoralis major intercostal muscles, ribs, and pleura and trace medially toward the sternum until the TTM comes into view lateral to the sternum and deep to the internal intercostal muscle as a hypoechoic band (Figure 1).
Identify the internal thoracic artery in this plane between the intercostal muscle and TTM to avoid vascular puncture and intra-arterial injection. To ensure constant visualization of the needle and prevent pericardial puncture, advance a blunt-tip, 21-gauge, 5- or 10-centimeter echogenic needle in plane from caudal to cranial direction. Place the needle tip between the TTM and the intercostal muscle (Figure 3). Confirm negative aspiration and correct placement in the TTP with 1–3 mL of sterile saline via hydrodissection followed by injection of 10–20 mL of 0.25% bupivacaine hydrochloride or ropivacaine 0.25% with 1–3 mg of preservative-free dexamethasone in 5 mL aliquots. Downward displacement of the pleura during injection further confirms correct deposition of local anesthetic.
The maximum dose of bupivacaine hydrochloride and ropivacaine is 2.5 mg/kg of ideal body weight. In addition to the local anesthetic’s analgesic component, hydrodissection of the TTP may allow for relief of entrapped terminal branches of the intercostal nerves, further alleviating pain.[7]
Approach
In our practice, we approach the plane of interest in a caudal to cranial fashion. Because the rib spaces can be small in this area, moving the ultrasound transducer cranially in the parasagittal plane helps us to identify the target by removing the inferior rib from the screen. This allows us to ensure that the needle is inserted in plane steep enough to reach the plane without bending or being inadvertently placed beneath the superior rib, where we cannot visualize the tip on ultrasound because of an acoustic shadow (Figure 3).
If rib spaces are narrow and limit the angle of the needle and appropriate visualization, we may use an out-of-plane approach. Place the high-frequency linear ultrasound probe in the same parasagittal orientation between ribs 3 and 4, and identify the TTP. Using a blunt-tip 21-gauge needle, approach the TTP in an out-of-plane fashion, using sterile saline for hydrodissection to further appreciate the tip of the needle. Given that the entirety of the needle cannot be appreciated and that the tip, even using hydrodissection, can be difficult to visualize, we do not recommend this approach because of the increased risk of pneumothorax, vascular puncture, and hematoma.
Dose and Volume
We have performed the block successfully with 10–20 mL of 0.25% bupivacaine or 0.25% ropivacaine. If the patient is having no other blocks and no intravenous local anesthetic administered, using a volume closer to 20 mL is preferable because it is a volume-dependent block. However, if the patient has a low body weight (less than 40 kilograms) or if other local anesthetics contribute to the total dose, 10 mL has provided effective analgesia in the past, anecdotally.
Block location and intercostal vasculature absorption influence its duration. It has the potential for biphasic absorption, with the initial phase occurring within five minutes because of the proximity of intercostal vasculature and the second, slower phase resulting from the surrounding subcutaneous fat absorbing the highly lipid soluble local anesthetic.[8]
See Figure 5 for clinical pearls regarding the TTBP.
Potential Complications
Adverse events to consider include:
- Pneumothorax
- Hemothorax
- Pericardial puncture, resulting in potential hemopericardium or pericardial injection
- Intravascular injection
- Local anesthetic systemic toxicity
- Damage to the internal thoracic artery
- Hematoma
- Infection
- Neural injury
Pecto-Intercostal Fascial Block
Alternative Methods
TTPB is not without its risks, including vascular and pericardial puncture, pneumothorax, LAST, infection, and nerve damage. A potentially safer alternative method is the pecto-intercostal fascial block (PIFB) in which the proceduralist injects local anesthetic in the fascial plane between the pectoralis major and the intercostal muscles.[9] Despite being a more superficial nerve block, PIFB also anesthetizes the anterior branches of the intercostal nerves that traverse through the intercostal muscles, but it is a novel block that requires additional research. However, several case reports suggest that PIFB may provide equivalent analgesia relative to TTPB for a variety of situations, including breast surgery, thymectomy, and sternotomy.
Technique
Place the patient in supine position with a linear probe placed in a longitudinal fashion just lateral to the sternum until the pectoralis major, ribs, and intercostal muscles are identified in the short axis. Confirm and avoid the location of the internal thoracic artery in a similar fashion when performing the TTPB. Advance a 21-gauge, 5 centimeter, blunt tip echogenic needle in plane under ultrasound guidance in a caudal to cranial direction, and place the tip between the pectoralis major and intercostal muscles. Hydrodissect with 1–2 mL aliquots of saline to confirm appropriate needle placement. Local anesthetic choice is proceduralist dependent and may be injected once negative aspiration is confirmed; 2–3 milliliters of 0.375% levobupivacaine with epinephrine 1:200,000 covers a dermatome[10] (Figure 5).
Liu et al reported T1–T6 coverage for poststernotomy analgesia with PIFB, but they did use three injection sites on each side with a total of 38 mL of ropivacaine 0.25% and decadron 3.8 mg injected.[11] Multiple injection sites may not be necessary; Jones et al described bilateral PIFB catheters placement with an initial bolus of 30 mL of bupivacaine 0.25% and ropivacaine 0.2% infusion at 8–10 mL/hr over the next four days in patients with myasthenia gravis receiving thymectomy.[12]
Although PIFB requires additional study, it offers the potential for a safer approach to anesthetizing the medial portion of the anterior chest wall.

Conclusion
As reliance on opioids as a sole analgesic option wanes, new regional anesthesia techniques that focus on fascial planes have gained prominence as critical components of multimodal analgesic regimens. Patients experiencing medial chest wall pain had not previously benefitted from regional anesthesia techniques and have largely depended on intravenous and oral opioids for pain control. Although techniques that block spinal nerves (paravertebral block) or proximal intercostal nerves (intercostal block) may also be effective at managing parasternal pain, they are limited by either the potential for sympathectomy with resulting hemodynamic changes or the need to perform multiple injections to properly anesthetize a large area.
Opioid consumption has known side effects, including nausea, vomiting, decreased bowel function, diminished ventilatory drive with hypercarbia, somnolence, and tolerance and may lead to problems with physical dependence or addiction. Non-opioid multimodal analgesic regimens may result in incomplete analgesia and may not be tolerated in various patient groups. Epidural and paravertebral blockade require strict timing of venous thromboembolism prophylaxis protocols and may be associated with spinal hematoma and neuraxial injuries. In the authors’ experience, TTPB requires no alteration of the anticoagulant regimen, although vascularity and block difficulty should be taken into account when making a decision.
Poorly managed acute pain may ultimately result in the development of chronic pain and the need for costly and stressful additional medical or surgical intervention. “The reported incidence of chronic pain after cardiac surgery varies from 21%–56% and is multifactorial in nature with a large neuropathic component.”[13] Therefore, the cardiac surgery population may be particularly vulnerable and warrant additional perioperative analgesic efforts.
Regional anesthesia techniques contribute to analgesia, limit reliance on opioids, treat neuropathic pain, and can prevent the development of chronic pain. With such a high incidence of chronic pain in the surgical population, TTPB proves to be a useful tool in the anesthesiologist’s arsenal.
References
- Miller M, Garg J, Salter B, et al. Feasibility of subcutaneous implantable cardioverter-defibrillator implantation with opioid sparing truncal plane blocks and deep sedation. J Cardiovasc Electrophysiol. 2018;30(1):141–148. https://doi.org/10.1111/jce.13750
- Murata H, Hida K, Hara T. Transverse thoracic muscle plane block. Reg Anesth Pain Med. 2016;41(3):411–412. https://doi.org/10.1097/aap.0000000000000374
- Mittnacht A, Shariat A, Weiner M, et al. Regional techniques for cardiac and cardiac-related procedures. J Cardiothorac Vasc Anesth. 2019;33(2):532–546. https://doi.org/10.1053/j.jvca.2018.09.017
- Thomas K, Sainudeen S, Jose S, Nadhari M, Macaire P. Ultrasound-guided parasternal block allows optimal pain relief and ventilation improvement after a sternal fracture. Pain Ther. 2016;5(1):115–122. https://doi.org/10.1007/s40122-016-0050-5
- Kirksey MA, Haskins SC, Cheng J, Liu SS. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: a systematic qualitative review. PLoS One. 2015;10(9):e0137312. https://doi.org/10.1371/journal.pone.0137312
- Ueshima H, Otake H. Addition of transversus thoracic muscle plane block to pectoral nerves block provides more effective perioperative pain relief than pectoral nerves block alone for breast cancer surgery. Br J Anaesth. 2017;118(3):439–443. https://doi.org/10.1093/bja/aew449
- Piraccini E, Biondi G, Byrne H, et al. Ultrasound guided transversus thoracic plane block, parasternal block and fascial planes hydrodissection for internal mammary post thoracotomy pain syndrome. Eur J Pain. 2018;22(9):1673–1677. https://doi.org/10.1002/ejp.1249
- McDonald S, Jacobsohn E, Kopacz D, et al. Parasternal block and local anesthetic infiltration with levobupivacaine after cardiac surgery with desflurane: the effect on postoperative pain, pulmonary function, and tracheal extubation times. Anesth Analg. 2005;100(1):25–32. https://doi.org/10.1213/01.ane.0000139652.84897.BD
- Hong B, Yoon SH, Youn AM, Kim BJ, Song S, Yoon Y. Thoracic interfascial nerve block for breast surgery in a pregnant woman: a case report. Korean J Anesthesiol. 2017;70(2):209–212. https://doi.org/10.4097/kjae.2017.70.2.209
- de la Torre PA, García PD, Alvarez SL, Miguel FJ, Pérez MF. A novel ultrasound-guided block: a promising alternative for breast analgesia. Aesthet Surg J. 2014;34(1):198–200. https://doi.org/10.1177/1090820X13515902
- Liu V, Mariano ER, Prabhakar C. Pecto-intercostal fascial block for acute poststernotomy pain. A A Pract. 2018;10(12):319–322. https://doi.org/10.1213/XAA.0000000000000697
- Jones J, Murin PJ, Tsui JH. Opioid free postoperatively using pecto-intercostal fascial block (PIFB) with multimodal analgesia (MMA) in a patient with myasthenia gravis underwent thymectomy via sternotomy. J Clin Anesth. 2020;59:32–33. https://doi.org/10.1016/j.jclinane.2019.06.009
- Dogan Baki E, Kavrut Ozturk N, Ayoglu R, Emmiler M, Karsli B, Uzel H. Effects of parasternal block on acute and chronic pain in patients undergoing coronary artery surgery. Semin Cardiothorac Vasc Anesth. 2015;20(3):205–212. https://doi.org/10.1177/1089253215576756
Leave a commentOrder by
Newest on top Oldest on top