View by publication date
(most recent on top)
Latest articles
How I Do It: Scalp Blocks for the Neuroanesthesiologist
Cite as: Dean C, Papangelou A. How I do it: scalp blocks for the neuroanesthesiologist. ASRA News 2021;46. https://doi.org/10.52211/asra110121.067
Achieving adequate pain control in craniotomy patients is an important anesthetic challenge. The understanding of the prevalence and characteristics of their pain experience has changed over the past 20–30 years. The historical view that patients have minimal pain after craniotomy, coupled with the idea that pain treatments can be dangerous, leaves many patients with inadequately treated pain.1
Postcraniotomy pain is most significant for 48 hours after surgery. The pain is thought to be somatic and has been described as a headache with superficial, generalized, and pounding or pulsating qualities. Despite some improvements in characterization, treatment options remain heterogeneous and inadequate in up to 60% of patients.1 Deciding how to treat this underrecognized and undertreated side effect has important implications for the postoperative course of therapy, management of complications, and patient experience.
Use of preincisional scalp blockade in the operating room is supported by many benefits, including improved hemodynamic stability, decreased anesthetic and opioid requirements, and enhanced postoperative analgesia. Substantial evidence supports blockade prior to pinning and incision to prevent significant hemodynamic changes.2
Elevated blood pressure and heart rate can lead to end-organ damage, which is an especially devastating neurologic injury during neurosurgical procedures, including aneurysm rupture prior to clipping. Limiting sedating medications, such as opioids, allows prompt postoperative neurologic assessment to identify neurologic injury. Our goal is to avoid untoward side effects of opioids, such as nausea, vomiting, respiratory depression, sedation, and hypercapnia, which are all potential signs of neurosurgical complications.
Properly executed scalp blockade benefits a patient’s analgesic course in the immediate postoperative period.2 Better hemodynamic and pain control, along with anesthetic and opioid-sparing properties, support the value of scalp blockade.
Anatomy
The scalp is innervated by the bilateral cranial and spinal nerves.3 Figures 1 and 2 illustrate two views of innervation of the head. Anterior forehead and scalp sensation is transmitted laterally by the supraorbital nerve and medially by the supratrochlear nerve. The two nerves combine to form the frontal nerve and continue as the ophthalmic division of the trigeminal nerve. The anterior temple and lateral forehead are innervated by the zygomaticotemporal nerve from the zygomatic nerve, a branch of the trigeminal nerve’s maxillary division. The posterior temple and anterior ear are innervated by the auriculotemporal nerve, a branch of the trigeminal nerve’s mandibular division. Sensation in the posterior ear and angle of the jaw is from the posterior branch of the great auricular nerve, which travels to the ventral rami of C2 and C3. The greater and lesser occipital nerves, which are branches of the C2 and C3 cervical nerves, innervate the posterior scalp.

Figure 1. Innervation of the head, anterior view5
Adapted with permission.
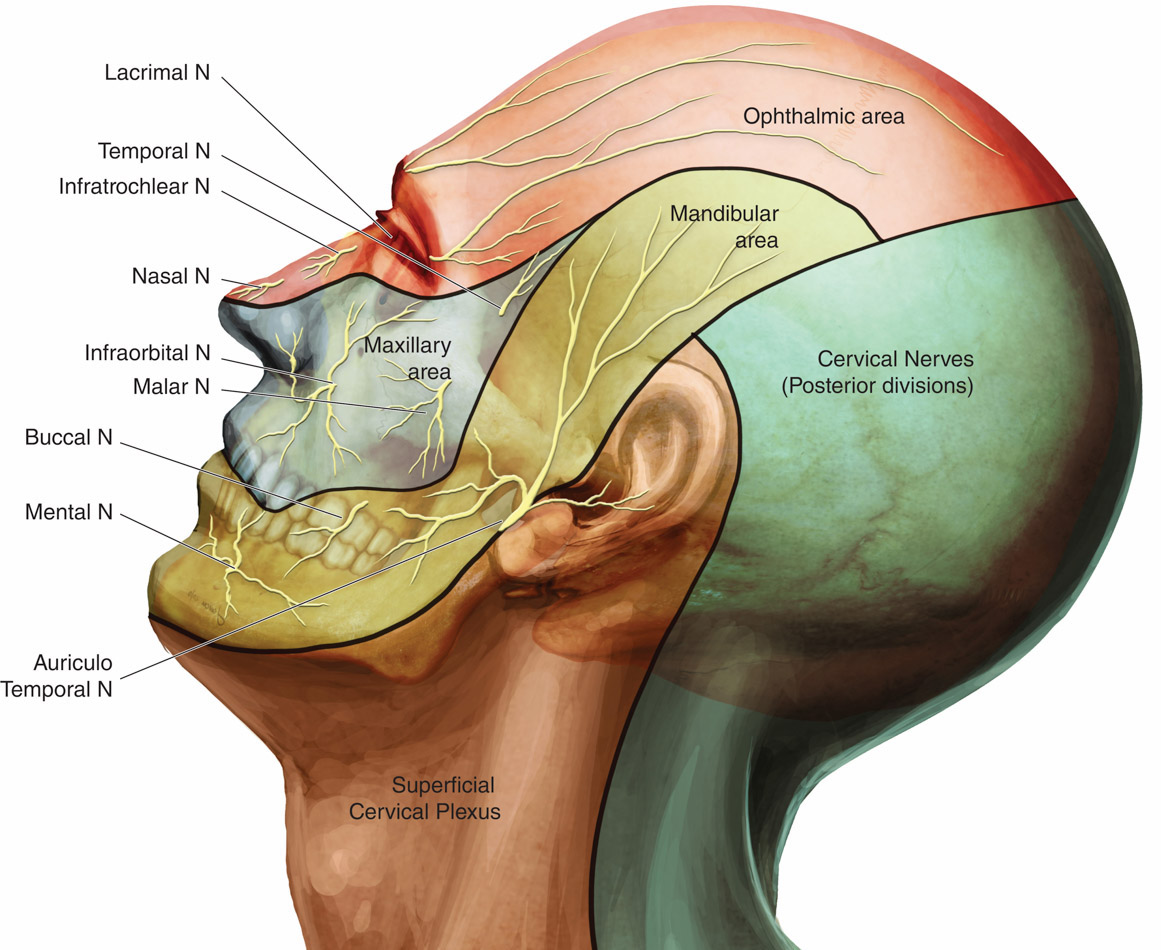
Figure 2. Innervation of the head, lateral view11
Used with permission.
Technique
We use a landmark technique targeting applicable nerves for the scalp blockade. With the patient in a neutral head position, identify the location of the supraorbital nerve by the supraorbital notch under the orbital ridge and the medial iris.4 If the notch is difficult to identify, the nerve is located on average 2.9 cm from the midline and 0.5 cm from the upper margin of the supraorbital rim.5 The supratrochlear nerve can be located 1.6 cm from the midline or 0.9–1.3 cm medial to the supraorbital nerve and 0.7 cm below the upper margin of the supraorbital rim.5
We use the index finger of the nondominant hand below the supraorbital rim to help protect the orbit from injury while injecting 1 mL of local anesthetic around each nerve, perpendicular to the skin (Figure 3). We use 0.5% bupivacaine, based on our evaluation of the literature.6

Figure 3. Scalp blockade injection
A 25-gauge needle is used to block the supratrochlear nerve (A) and the supraorbital nerve (B) along the supraorbital rim. The nondominant hand is used to protect the orbit.
To reach the many branches in variable patterns of the zygomaticotemporal nerve, deep and superficial injections are required. Advance the needle along the bone of the lateral orbital rim 1–1.7 cm posterior to the frontozygomatic suture and 2.2 cm above upper margin of zygomatic arch, injecting 2 mL of local anesthetic.6 Anesthetize the auriculotemporal nerve 1–1.5 cm anterior to the tragus with 1 mL superficially to prevent inadvertent injection to the facial nerve. Alternatively, inject the auriculotemporal nerve 1–1.5 cm anterior to the superior pinna. Target the posterior branch of the great auricular nerve 1.5 cm posterior to the pinna at the level of the tragus with 1 mL of local anesthetic. Perform the remaining injections along the superior nuchal line between the occipital protuberance and the mastoid process. A total of 5 mL of local anesthetic anesthetizes the lesser and greater occipital nerves 7 cm and 4 cm lateral to the occipital protuberance.7 Careful aspiration is always recommended prior to injection for the greater occipital nerve because of its proximity to the occipital artery. If the occipital artery is palpable, an injection in the proximity of the vessel will yield a successful block. All injections are summarized in Table 1.

Clinical Applications
Preoperative assessment for appropriateness of scalp block is necessary. The timing and extent of the scalp block should be coordinated with the neurosurgery team. It is not necessary to execute blockade of all the aforementioned nerves, particularly in the asleep craniotomy where incisions are at different locations on the cranium. For awake craniotomy cases, we perform bilateral scalp blockade in the preoperative holding area. Our preference is to perform the scalp block without pharmacologic anxiolysis to prevent sedative and cognitive effects. Expectation management is key to successful patient tolerance of the procedure.
Blockade of the zygomaticotemporal nerve is difficult and usually incomplete because of its extensive branching and a variable course.8 Neurosurgery frequently requires injections of local anesthesia into the temporalis fascia during the dissection at the start of the awake craniotomy. From discussions with many leading neuroanesthesiologists interested in scalp blockade, regional infiltration is almost universally required to obtain adequate analgesia.9 We believe this to be a topic that warrants additional research.
Contraindications to regional anesthesia include patient refusal, local anesthetic allergy, and infection at the injection site. Complications include local anesthetic systemic toxicity, hematoma, allergic reaction, orbital injury, arterial injury, inadvertent subarachnoid blockade, and facial nerve paralysis. Relevant arteries include the superficial temporal artery (STA) during the auriculotemporal nerve block and the occipital artery during the greater occipital nerve block. When injury to the STA is particularly unwelcome, as in STA direct revascularization or bypass, the auriculotemporal nerve block can be omitted or performed postoperatively. A facial nerve palsy is an unwanted, albeit temporary, side effect of the auriculotemporal block when the injection is too deep. When a fascial nerve palsy must be avoided or if an incision will not extend inferiorly to the level of the helix of the ear, the auriculotemporal nerve can be blocked at the top of the pinna rather than the tragus. This will anesthetize the superior distribution of the nerve but may be insufficient for an awake craniotomy incision.10 The potential for inadvertent subarachnoid blockade exists, especially in patients with skull defects, including prior craniectomy.
Conclusion
Landmark-guided scalp blockade is a safe and effective analgesic technique for craniotomy patients. The benefits of successful scalp blockade extend beyond analgesia to include improved hemodynamic stability, and opioid-sparing properties.

Cassandra Dean, MD, is an assistant professor of anesthesiology at Emory University Hospital in Atlanta, GA.

Alexander Papangelou, MD, is an associate professor of anesthesiology and division chief of neuroanesthesiology at Emory University Hospital in Atlanta, GA.
References
- De Benedittis G, Lorenzetti A, Migliore M, et al. Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery. 1996;38(3):466–9; discussion 469–70. https://doi.org/10.1097/00006123-199603000-00008
- Carella M, Tran G, Bonhomme VL, et al. Influence of levobupivacaine regional scalp block on hemodynamic stability, intra- and postoperative opioid consumption in supratentorial craniotomies: a randomized controlled trial. Anesth Analg. 2021;132(2):500–11. https://doi.org/10.1213/ANE.0000000000005230
- Kemp WJ, 3rd, Tubbs RS, Cohen-Gadol AA. The innervation of the scalp: a comprehensive review including anatomy, pathology, and neurosurgical correlates. Surg Neurol Int. 2011;2:178. https://doi.org/10.4103/2152-7806.90699
- Cuzalina AL, Holmes JD. A simple and reliable landmark for identification of the supraorbital nerve in surgery of the forehead: an in vivo anatomical study. J Oral Maxillofac Surg. 2005;63(1):25–7. https://doi.org/10.1016/j.joms.2004.04.026
- Jeong SM, Park KJ, Kang SH, et al. Anatomical consideration of the anterior and lateral cutaneous nerves in the scalp. J Korean Med Sci. 2010;25(4):517–22. https://doi.org/10.3346/jkms.2010.25.4.517
- Papangelou A, Radzik BR, Smith T, et al. A review of scalp blockade for cranial surgery. J Clin Anesth. 2013;25(2):150–9. https://doi.org/10.1016/j.jclinane.2012.06.024
- Tubbs RS, Salter EG, Wellons JC, et al. Landmarks for the identification of the cutaneous nerves of the occiput and nuchal regions. Clin Anat. 2007;20(3):235–8. https://doi.org/10.1002/ca.20297
- Janis JE, Hatef DA, Thakar H, et al. The zygomaticotemporal branch of the trigeminal nerve: part II. Anatomical variations. Plast Reconstr Surg. 2010;126(2):435–42. https://doi.org/10.1097/PRS.0b013e3181e094d7
- Girvin JP. Neurosurgical considerations and general methods for craniotomy under local anesthesia. Int Anesthesiol Clin. 1986;24(3):89–114. https://doi.org/10.1097/00004311-198602430-00010
- Lee HJ, Choi YJ, Lee KW, et al. Positional patterns among the auriculotemporal nerve, superficial temporal artery, and superficial temporal vein for use in decompression treatments for migraine. Sci Rep. 2018;8(1):16539. https://doi.org/10.1038/s41598-018-34765-1
- Sola C, Choquet O, Capdevila X. Nerve blocks of the face. https://www.nysora.com/techniques/head-and-neck-blocks/nerve-blocks-face. Accessed October 22, 2021.
Most popular articles
How I Do It: Scalp Blocks for the Neuroanesthesiologist
Cite as: Dean C, Papangelou A. How I do it: scalp blocks for the neuroanesthesiologist. ASRA News 2021;46. https://doi.org/10.52211/asra110121.067
Achieving adequate pain control in craniotomy patients is an important anesthetic challenge. The understanding of the prevalence and characteristics of their pain experience has changed over the past 20–30 years. The historical view that patients have minimal pain after craniotomy, coupled with the idea that pain treatments can be dangerous, leaves many patients with inadequately treated pain.1
Postcraniotomy pain is most significant for 48 hours after surgery. The pain is thought to be somatic and has been described as a headache with superficial, generalized, and pounding or pulsating qualities. Despite some improvements in characterization, treatment options remain heterogeneous and inadequate in up to 60% of patients.1 Deciding how to treat this underrecognized and undertreated side effect has important implications for the postoperative course of therapy, management of complications, and patient experience.
Use of preincisional scalp blockade in the operating room is supported by many benefits, including improved hemodynamic stability, decreased anesthetic and opioid requirements, and enhanced postoperative analgesia. Substantial evidence supports blockade prior to pinning and incision to prevent significant hemodynamic changes.2
Elevated blood pressure and heart rate can lead to end-organ damage, which is an especially devastating neurologic injury during neurosurgical procedures, including aneurysm rupture prior to clipping. Limiting sedating medications, such as opioids, allows prompt postoperative neurologic assessment to identify neurologic injury. Our goal is to avoid untoward side effects of opioids, such as nausea, vomiting, respiratory depression, sedation, and hypercapnia, which are all potential signs of neurosurgical complications.
Properly executed scalp blockade benefits a patient’s analgesic course in the immediate postoperative period.2 Better hemodynamic and pain control, along with anesthetic and opioid-sparing properties, support the value of scalp blockade.
Anatomy
The scalp is innervated by the bilateral cranial and spinal nerves.3 Figures 1 and 2 illustrate two views of innervation of the head. Anterior forehead and scalp sensation is transmitted laterally by the supraorbital nerve and medially by the supratrochlear nerve. The two nerves combine to form the frontal nerve and continue as the ophthalmic division of the trigeminal nerve. The anterior temple and lateral forehead are innervated by the zygomaticotemporal nerve from the zygomatic nerve, a branch of the trigeminal nerve’s maxillary division. The posterior temple and anterior ear are innervated by the auriculotemporal nerve, a branch of the trigeminal nerve’s mandibular division. Sensation in the posterior ear and angle of the jaw is from the posterior branch of the great auricular nerve, which travels to the ventral rami of C2 and C3. The greater and lesser occipital nerves, which are branches of the C2 and C3 cervical nerves, innervate the posterior scalp.

Figure 1. Innervation of the head, anterior view5
Adapted with permission.

Figure 2. Innervation of the head, lateral view11
Used with permission.
Technique
We use a landmark technique targeting applicable nerves for the scalp blockade. With the patient in a neutral head position, identify the location of the supraorbital nerve by the supraorbital notch under the orbital ridge and the medial iris.4 If the notch is difficult to identify, the nerve is located on average 2.9 cm from the midline and 0.5 cm from the upper margin of the supraorbital rim.5 The supratrochlear nerve can be located 1.6 cm from the midline or 0.9–1.3 cm medial to the supraorbital nerve and 0.7 cm below the upper margin of the supraorbital rim.5
We use the index finger of the nondominant hand below the supraorbital rim to help protect the orbit from injury while injecting 1 mL of local anesthetic around each nerve, perpendicular to the skin (Figure 3). We use 0.5% bupivacaine, based on our evaluation of the literature.6

Figure 3. Scalp blockade injection
A 25-gauge needle is used to block the supratrochlear nerve (A) and the supraorbital nerve (B) along the supraorbital rim. The nondominant hand is used to protect the orbit.
To reach the many branches in variable patterns of the zygomaticotemporal nerve, deep and superficial injections are required. Advance the needle along the bone of the lateral orbital rim 1–1.7 cm posterior to the frontozygomatic suture and 2.2 cm above upper margin of zygomatic arch, injecting 2 mL of local anesthetic.6 Anesthetize the auriculotemporal nerve 1–1.5 cm anterior to the tragus with 1 mL superficially to prevent inadvertent injection to the facial nerve. Alternatively, inject the auriculotemporal nerve 1–1.5 cm anterior to the superior pinna. Target the posterior branch of the great auricular nerve 1.5 cm posterior to the pinna at the level of the tragus with 1 mL of local anesthetic. Perform the remaining injections along the superior nuchal line between the occipital protuberance and the mastoid process. A total of 5 mL of local anesthetic anesthetizes the lesser and greater occipital nerves 7 cm and 4 cm lateral to the occipital protuberance.7 Careful aspiration is always recommended prior to injection for the greater occipital nerve because of its proximity to the occipital artery. If the occipital artery is palpable, an injection in the proximity of the vessel will yield a successful block. All injections are summarized in Table 1.

Clinical Applications
Preoperative assessment for appropriateness of scalp block is necessary. The timing and extent of the scalp block should be coordinated with the neurosurgery team. It is not necessary to execute blockade of all the aforementioned nerves, particularly in the asleep craniotomy where incisions are at different locations on the cranium. For awake craniotomy cases, we perform bilateral scalp blockade in the preoperative holding area. Our preference is to perform the scalp block without pharmacologic anxiolysis to prevent sedative and cognitive effects. Expectation management is key to successful patient tolerance of the procedure.
Blockade of the zygomaticotemporal nerve is difficult and usually incomplete because of its extensive branching and a variable course.8 Neurosurgery frequently requires injections of local anesthesia into the temporalis fascia during the dissection at the start of the awake craniotomy. From discussions with many leading neuroanesthesiologists interested in scalp blockade, regional infiltration is almost universally required to obtain adequate analgesia.9 We believe this to be a topic that warrants additional research.
Contraindications to regional anesthesia include patient refusal, local anesthetic allergy, and infection at the injection site. Complications include local anesthetic systemic toxicity, hematoma, allergic reaction, orbital injury, arterial injury, inadvertent subarachnoid blockade, and facial nerve paralysis. Relevant arteries include the superficial temporal artery (STA) during the auriculotemporal nerve block and the occipital artery during the greater occipital nerve block. When injury to the STA is particularly unwelcome, as in STA direct revascularization or bypass, the auriculotemporal nerve block can be omitted or performed postoperatively. A facial nerve palsy is an unwanted, albeit temporary, side effect of the auriculotemporal block when the injection is too deep. When a fascial nerve palsy must be avoided or if an incision will not extend inferiorly to the level of the helix of the ear, the auriculotemporal nerve can be blocked at the top of the pinna rather than the tragus. This will anesthetize the superior distribution of the nerve but may be insufficient for an awake craniotomy incision.10 The potential for inadvertent subarachnoid blockade exists, especially in patients with skull defects, including prior craniectomy.
Conclusion
Landmark-guided scalp blockade is a safe and effective analgesic technique for craniotomy patients. The benefits of successful scalp blockade extend beyond analgesia to include improved hemodynamic stability, and opioid-sparing properties.

Cassandra Dean, MD, is an assistant professor of anesthesiology at Emory University Hospital in Atlanta, GA.

Alexander Papangelou, MD, is an associate professor of anesthesiology and division chief of neuroanesthesiology at Emory University Hospital in Atlanta, GA.
References
- De Benedittis G, Lorenzetti A, Migliore M, et al. Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery. 1996;38(3):466–9; discussion 469–70. https://doi.org/10.1097/00006123-199603000-00008
- Carella M, Tran G, Bonhomme VL, et al. Influence of levobupivacaine regional scalp block on hemodynamic stability, intra- and postoperative opioid consumption in supratentorial craniotomies: a randomized controlled trial. Anesth Analg. 2021;132(2):500–11. https://doi.org/10.1213/ANE.0000000000005230
- Kemp WJ, 3rd, Tubbs RS, Cohen-Gadol AA. The innervation of the scalp: a comprehensive review including anatomy, pathology, and neurosurgical correlates. Surg Neurol Int. 2011;2:178. https://doi.org/10.4103/2152-7806.90699
- Cuzalina AL, Holmes JD. A simple and reliable landmark for identification of the supraorbital nerve in surgery of the forehead: an in vivo anatomical study. J Oral Maxillofac Surg. 2005;63(1):25–7. https://doi.org/10.1016/j.joms.2004.04.026
- Jeong SM, Park KJ, Kang SH, et al. Anatomical consideration of the anterior and lateral cutaneous nerves in the scalp. J Korean Med Sci. 2010;25(4):517–22. https://doi.org/10.3346/jkms.2010.25.4.517
- Papangelou A, Radzik BR, Smith T, et al. A review of scalp blockade for cranial surgery. J Clin Anesth. 2013;25(2):150–9. https://doi.org/10.1016/j.jclinane.2012.06.024
- Tubbs RS, Salter EG, Wellons JC, et al. Landmarks for the identification of the cutaneous nerves of the occiput and nuchal regions. Clin Anat. 2007;20(3):235–8. https://doi.org/10.1002/ca.20297
- Janis JE, Hatef DA, Thakar H, et al. The zygomaticotemporal branch of the trigeminal nerve: part II. Anatomical variations. Plast Reconstr Surg. 2010;126(2):435–42. https://doi.org/10.1097/PRS.0b013e3181e094d7
- Girvin JP. Neurosurgical considerations and general methods for craniotomy under local anesthesia. Int Anesthesiol Clin. 1986;24(3):89–114. https://doi.org/10.1097/00004311-198602430-00010
- Lee HJ, Choi YJ, Lee KW, et al. Positional patterns among the auriculotemporal nerve, superficial temporal artery, and superficial temporal vein for use in decompression treatments for migraine. Sci Rep. 2018;8(1):16539. https://doi.org/10.1038/s41598-018-34765-1
- Sola C, Choquet O, Capdevila X. Nerve blocks of the face. https://www.nysora.com/techniques/head-and-neck-blocks/nerve-blocks-face. Accessed October 22, 2021.

Leave a commentOrder by
Newest on top Oldest on top