Chest Wall Pain in a Patient With Lung Cancer Following Radiation Treatment: A Problem Based Learning Discussion
A 69-year-old male, recent former smoker, with a past medical history of chronic obstructive pulmonary disease, hypertension, and squamous cell non-small cell lung carcinoma (NSCLC) status post chemoradiotherapy completed approximately one year prior, presents to his oncologist with new right-sided thoracic chest pain. The pain is located just underneath his right armpit and refers to his back. The patient describes the pain as constant, and it wakes him up from his sleep at times. He rates the pain as a 10/10 at its maximum. He is currently taking acetaminophen over the counter for his pain, which provides minimal benefit. He denies any history of recent trauma. His other comorbidities are well managed, and he tolerates his medications (metoprolol succinate, omeprazole, and sacubitril-valsartan) without issue.
In regard to his cancer treatment, he received radiation therapy to his right lung at a dose of 60 Gy followed by combination chemotherapy of carboplatin and paclitaxel for six weeks. After completion of his chemoradiotherapy, he was placed on durvalumab for maintenance therapy but only tolerated it for three months due to worsening dyspnea.
There are no recent pertinent laboratory studies. He receives a computed tomography (CT) chest every three months for active surveillance. His most recent CT chest scan demonstrated increased right upper lobe fibrosis but showed no evidence of new lesions or increased size of previously known lesions.
The patient does have a history of back pain, which has been treated in the past with cervical and thoracic trigger point injections as well as gabapentin. He reports that he has tried opioids in the past for pain, but they caused significant mood changes which led to cessation of medication. The review of systems is consistent with fatigue, dyspnea, and shortness of breath. He notes that his dyspnea and shortness of breath are at their baseline and have not worsened recently. He denies any recent fever, weight loss, or night sweats. A physical examination was notable for tenderness to palpation underneath his right axilla; otherwise, his physical examination was unremarkable.
Questions
1. Given this patient’s medical history and review of systems, what is your differential diagnosis?
With any patient presentation, it is important to keep the differential diagnosis broad. A differential diagnosis should include, but not be limited to, tumor recurrence/new malignancy, intercostal neuralgia, rib fracture, radiation induced lung injury (RILI), myositis, pneumothorax, and esophagitis.
Tumor recurrence or a new malignancy is the most important to rule out initially. NSCLC is notorious for invading into the chest wall, resulting in acute chest wall pain.1 Development of a new malignancy, such as osteosarcoma, is possible. While this is a rare late complication of external radiotherapy (7-10 years postradiation), it should still be considered.2 Radiation-induced damage can include myositis, specifically inflammation of the intercostal muscles, which can mimic tumor invasion on imaging.3 In addition to myositis, radiation can cause intercostal neuralgia or nociceptive pain. Rib fractures are also a known complication of radiation therapy associated with edema and osteosclerosis. Typically, larger volumes of chest wall radiation (i.e., doses greater than 30-50 Gy) are associated with rib fractures.4 RILI includes both pneumonitis and fibrosis. The incidence of radiation pneumonitis largely is dose dependent, with higher doses having a higher incidence of RILI.5 Pulmonary fibrosis can lead to a pneumothorax and present with acute chest pain, as well as shortness of breath.6 Due to the location of the patient’s pain, esophagitis is less likely; however, radiation therapy is associated with an increased risk for radiation-induced and infectious esophagitis. Presenting symptoms typically include chest pain, dysphagia, and odynophagia.
2. The patient’s oncologist is concerned about the potential for new metastatic lesions and is planning further work-up. What initial imaging studies would be recommended?
When evaluating chest wall pain in a patient with a history of lung cancer postradiation, choosing the appropriate imaging modality is crucial for accurate diagnosis and management. CT is the primary imaging modality for diagnosis, staging, and follow-up of most thoracic cavity tumors, including lung cancer. CT allows for detailed anatomical information, which is essential for staging and distinguishing normal postradiation changes from complications such as infection or recurrence. Compared to positron emission tomography (PET) scan, CT is limited at differentiating benign and malignant lesions, in addition to assessing metabolic activity.7,8 Therefore, PET is an important tool for refining diagnosis and staging in patients with possible lung cancer, providing metabolic data of 18F-fluorodeoxyglucose (FDG) cellular uptake in addition to morphologic CT data. PET is also superior to CT for locoregional lymph node staging. Limitations of PET include false-positive results due to inflammatory lesions and false-negative results in small or low-metabolic neoplasms.8 Magnetic resonance imaging (MRI) is an important adjunctive imaging modality in thoracic oncologic imaging and is used as a problem-solving tool to assess for chest wall invasion, intraspinal extension, and cardiac or vascular invasion. MRI provides improved soft tissue contrast compared to CT. However, the physical properties of the lungs and mediastinum create unique challenges for lung MRI, and its role in staging bronchogenic carcinoma remains limited.9 Ultimately, the patient’s oncologist decided to order a combined PET/CT due to his extensive past medical history and concerning symptoms.
3. The patient notes he will be unable to tolerate the pain while waiting to schedule and get scan results. As noted above, he states he is taking acetaminophen three to four times a day, yet he is experiencing excruciating pain. What is the World Health Organization (WHO) stepladder approach to manage cancer pain? What are some treatment options for potential neuropathic pain? What should the oncologist’s next step be in managing the patient’s pain based on the WHO stepladder?
The WHO stepladder approach used to be a three-step process to manage cancer pain, but recently it has been updated. It is now a four-step process as seen in the figure below.


For further specifics that are beyond the scope of this discussion on the WHO stepladder, please see prior problem-based learning discussions on cancer-associated pain.
Given this patient’s history of intolerance to oral opioids, after shared decision making, it was decided to trial a low-dose buprenorphine transdermal patch at 5 mcg/hr. His oncologist recommended continued use of acetaminophen as well as starting gabapentin for management of neuropathic pain.
4. Other than potential metastatic lesions, what other potential radiographic findings could be expected with his presenting symptoms and history of squamous cell carcinoma postradiation?
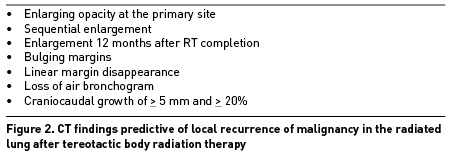
Acute phase RILI demonstrates gradual onset of ground-glass opacities that are within the previous radiation treatment area. These opacities may have a heterogeneous appearance acutely but can appear as lung consolidations chronically. These areas of fibrosis can breach typical anatomic boundaries, such as pleural fissures (whereas infectious opacities will adhere to anatomic boundaries). The fibrotic changes of RILI can have a chronic mass-like appearance that can be difficult to distinguish from tumor recurrence. The table below includes CT findings that are predictive of local recurrence of malignancy in radiated lungs post stereotactic body radiation therapy (SBRT).
CT studies are particularly sensitive to detecting postradiation changes; however, they are limited in their ability to differentiate infection, recurrence, and fibrosis. PET/CT is better at differentiating fibrosis versus tumor recurrence, although it is not without limitations. FDG, a glucose analog used in PET/CT imaging, will show increased uptake in the setting of infection, inflammation, and tumor recurrence. FDG uptake is expected to be increased in radiation pneumonitis, potentially up to six months following radiation treatment.10 Lastly, RILI can result in cavitary lesions. Given the diagnostic difficulties associated with differentiating RILI compared to other diseases, we recommend the revised RECIST 1.1 study to learn more about high-risk features associated with tumor recurrence.11
(Adapted from Hanania, AN et al. Radiation-induced lung injury: assessment and management. Chest. 2019;156:150. https://doi.org/10.1016/j.chest.2019.03.033)
5. Results of the patient’s combined PET/CT revealed evidence of a decrease in size and metabolic activity of the previously treated right upper lobe mass and a new hypermetabolic lytic lesion identified in the posterior lateral right sixth rib, concerning osseous metastases. Potential further treatment options for his cancer were planned to be discussed with his oncology team and are beyond the scope of this discussion. In regard to his chest pain likely from the new osseous metastatic 6th rib lesion, what interventional pain techniques would potentially be of benefit to the patient?
There are two interventional pain procedures of note with regard to treating the new osseous metastatic 6th rib lesion. One option is a fluoroscopic intercostal nerve block, which involves depositing a local anesthetic agent (such as lidocaine or bupivacaine) in the 6th subcostal groove to essentially “numb” that intercostal level for immediate, lasting pain relief. Another option is ultrasound or fluoroscopic intercostal neurolysis, which aims to destroy the intercostal nerve via various modalities, such as radiofrequency ablation, phenol, or alcohol.
6. The patient was referred to an interventional pain physician and was scheduled for an intercostal nerve block. What are some of the important anatomical factors of the intercostal nerves to keep in mind when performing the procedure?
The intercostal nerves arise from the anterior rami of the thoracic spinal nerves T1 to T11. These nerves traverse laterally behind the sympathetic trunk and enter the intercostal space. They run parallel to the subcostal groove, which is the inferior border of the associated rib. Surrounding the intercostal nerve is the innermost intercostal muscle and the internal intercostal muscle. Coursing with the nerve is the intercostal artery and vein. Just deep to the innermost intercostal muscle is the parietal pleura. Based on these structures, it is vital to have an in-depth knowledge of the intercostal space to avoid complications during needle driving and medication administration.
7. What are some of the potential complications of an intercostal nerve block? What imaging modalities, if any, are indicated for an intercostal nerve block to limit complications?
The most catastrophic complication for intercostal nerve blocks is pneumothorax. The rate for pneumothorax following intercostal nerve blocks is variable; however, one notable study demonstrated that the incidence for pneumothorax per individual intercostal nerve blocked was 1.4%.12 Of note, there are also case reports of inadvertent spinal anesthesia due to dural sacs protruding laterally from the vertebral foramen.
Anatomic landmark-guided procedures have been outlined and can be performed safely. However, appropriate use of ultrasound or fluoroscopic guidance can minimize the risk of intravascular injection and pneumothorax. These techniques also allow for injections closer to midline, increasing the likelihood that the lateral branch is anesthetized properly.13 As always, there is a risk of bleeding and infection, and thus nerve blocks are recommended to be performed under sterile technique and with special attention to patients who receiving anticoagulation or with a history of coagulopathy.
References
- Detterbeck FC, Zelman S, Diekemper R, Addrizzo-Harris D, Alberts W.M. Executive summary: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):7S-37S. https://doi.org/10.1378/chest.12-2377
- Ninomiya H, Miyoshi T, Shirakusa T, Shiraishi T, Yamamoto N, Nabeshima K. Postradiation sarcoma of the chest wall: report of two cases. Surg Today. 2006;36(12):1101–4. https://doi.org/10.1007/s00595-004-3300-9
- Tomita H, Kita T, Hayashi K, Kosuda S. Radiation-induced myositis mimicking chest wall tumor invasion in two patients with lung cancer: a PET/CT study. Clin Nucl Med. 2012 Feb;37(2):168–9. https://doi.org/10.1097/RLU.0b013e3181d6249f
- Bongers EM, Haasbeek CJ, Lagerwaard FJ, Slotman BJ, Senan S. Incidence and risk factors for chest wall toxicity after risk-adapted stereotactic radiotherapy for early-stage lung cancer. J Thorac Oncol. 2011 Dec;6(12):2052–7. https://doi.org/10.1097/JTO.0b013e3182307e74
- Barriger RB, Forquer JA, Brabham JG, Andolino DL, Shapiro RH, Henderson MA, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2012;82:457. https://doi.org/10.1016/j.ijrobp.2010.08.056
- Epstein DM, Littman P, Gefter WB, Miller WT, Raney RB. Radiation‐induced pneumothorax. Med Pediatr Oncol. 1983;11(2):122–4. https://doi.org/10.1002/mpo.2950110210
- Strange CD, Shroff GS, Truong MT, Nguyen QN, Vlahos I, Erasmus JJ. Imaging of the post-radiation chest in lung cancer. Clin Radiol. 2022 Jan;77(1):19–30. https://doi.org/10.1016/j.crad.2021.04.013
- Stamatis G. Staging of lung cancer: the role of noninvasive, minimally invasive and invasive techniques. Eur Respir J. 2015 Aug;46(2):521–31. https://doi.org/10.1183/09031936.00126714
- Sim AJ, Kaza E, Singer L, Rosenberg SA. A review of the role of MRI in diagnosis and treatment of early stage lung cancer. Clin Transl Radiat Oncol. 2020 Jun 6;24:16–22. https://doi.org/10.1016/j.ctro.2020.06.002
- Strange TA, Erasmus LT, Ahuja J, Agrawal R, Shroff GS, Truong MT, Strange CD. Spectrum of imaging patterns of lung cancer following radiation therapy. Diagnostics (Basel). 2023 Oct 23;13(20):3283. https://doi.org/10.3390/diagnostics13203283
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009 Jan;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026
- Shanti CM, Carlin AM, Tyburski JG. Incidence of pneumothorax from intercostal nerve block for analgesia in rib fractures. J Trauma. 2001 Sep;51(3):536–9. https://doi.org/10.1097/00005373-200109000-00019
- Glenesk NL, Rahman S, Lopez PP. Anatomy, thorax, intercostal nerves [Updated 2023 May 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538238/
Leave a commentOrder by
Newest on top Oldest on top