Simulation Models in Ultrasound-Guided Regional Anesthesia
Ultrasound-guided regional anesthesia (UGRA) is difficult to learn and takes many years to attain expertise. Anesthetic trainees lack confidence,1 find interpretation of ultrasound challenging,2 display wide variability in UGRA performance,3 and may expose patients to repeated attempts, pain, and harm.4 In a study scanning volunteers’ axillae, trainees committed three times more errors than experts. One out of six errors were sentinel errors: events that represent a serious deviation from optimal performance, jeopardize outcome, or harm patients.4
Simulation will never replace reality but should at least allow for the repeated, deliberate practice of salient aspects of a procedure and provide the same sensory feedback as experienced with patients.
In the United Kingdom (UK), anesthetists first learn ultrasound and needle procedural skills on patients, an approach at odds with the UK Department of Health’s recommendation that health professionals “should learn skills in a simulation environment . . . before undertaking them in supervised clinical practice.”5 The Royal College of Anaesthetists’ training curriculum and the Association of Anaesthetists’ guide to ultrasound training broadly define procedural skills but do not mention simulation.
Our experience is that more anesthesiologists and surgeons are aware of the benefits of regional anesthesia, yet training resources are limited. Medical education remains entrenched within the classical Halstedian approach. Halsted was a surgeon at the John Hopkins Hospital who established a surgical training program that focused on knowledge of the basic sciences, research, and extensive clinical exposure. His principles have been retained in many institutions but are increasingly seen as not suitable for current and future practice. The new safety culture has identified that training on the job using a “see one, do one, teach one” approach exposes patients to risk.
Additionally, providers using that approach take longer to gain sufficient clinical exposure to attain a basic level of proficiency. Average case numbers within training for anesthesiologists are falling year over year.6 Although case numbers do not align precisely to level of proficiency, fewer cases results in a challenging context in which to deliver training. To meet training needs, simulation has become essential.
Clinicians need to define which type of simulator and educational technique improves training in a safe environment. Simulation will never replace reality but should at least allow for the repeated, deliberate practice of salient aspects of a procedure and provide the same sensory feedback as experienced with patients. The properties of an ideal simulator are highlighted in Figure 1.
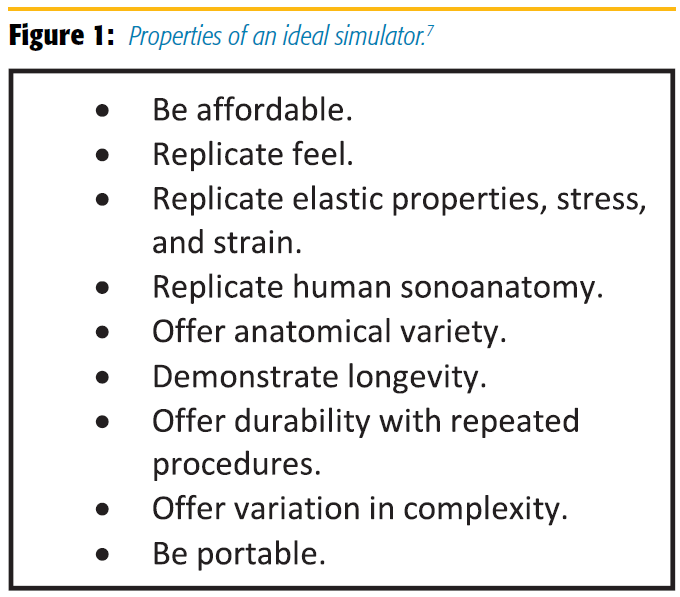
Simulators used in regional anesthesia training include plastic phantoms, gelatin or agar models, tofu, pork with embedded tendons, cadavers, and immersive technology, all of which have advantages and disadvantages.
Blue Phantom
Commercially available plastic simulators replicate a number of body parts for the simulation of ultrasound-guided procedures.8 Advantages include portability, long shelf life, no infection issues, relative durability, and reusability. Disadvantages include cost, fixed targets, visible needle tracks, and non-tissue like haptics. Needle visibility is generally better than in patients.
Gelatin and Agar
Gelatin- and agar-based models are easy to make and fulfill many of the ideal model criteria. Multiple targets can be imbedded in a model, including starch blocks, raisins, tubing, and wires. The models are useful for learning basic hand-eye coordination and may accelerate learning.9 Three-dimensional (3D) printing with gelatin offers the prospect of models with greater anatomical accuracy detail and complexity.10
Advantages include low price, wide availability of ingredients, easy preparation, portability, a large scanning surface for embedding several targets. Models are reproducible, may be clear or colored, manufactured to any size and shape required, and stored in a refrigerator for up to two weeks.7
The principal disadvantage is the uniform appearance on sonography unless additives such as husk or corn flour are used. Needle tracts are often visible and may mimic the needle, producing a double shadow. Agar is not universally available, and gelatin seeps water over time. Models can be easily damaged.
Tofu
Tofu is a vegetable-derived protein that, similar to gelatin and agar models, facilitates needle manipulation and target visualization for the novice. The advantages and disadvantages of tofu are similar to those of gelatin and agar.
Silicone
Silicone-based models incorporate electrical components and equipment for pulsatile flow identifiable via Doppler imaging.11 This model produces a sound when the needle makes contact with the targeted structure. The model comes preformed with a structure that shows pulsatile flow. Its advantages are absence of infection, a long shelf life, and portability. Disadvantages include persistence of needle tracts, small scanning area, and high cost. The preformed shape and size and embedded target structure offer no versatility. Additionally, the model requires a power source.
Animal Tissue
Shoulder of pork and leg of lamb are better simulators than chicken or turkey breast. Preparation entails deodorization by soaking in 70% alcohol overnight in a refrigerator inside a plastic bag. To replicate the size and shape of a peripheral nerve, a bovine tendon 1 cm in diameter is inserted through the muscle layers. The whole phantom is then wrapped up in a transparent film and stored at 4°C until use.12 Tendon is used instead of nerve because nerves are generally not available for purchase. A similar technique is applied to the preparation of leg of lamb, whereby a softened Achilles tendon is inserted through the specimen to model a nerve.
Advantages of tissue phantoms include wide availability and satisfactory appearance of muscle, fascia, bone, and the needle. Needle tracts are less apparent than with plastic models. The tactile feedback is also more realistic.
Tissue phantoms, however, have a shelf life of only a few days and present an infection risk. Air trapping can occur, limiting visibility of targets. Repeated injection of fluid is possible, but the tissue distorts and needling is limited.
Cadavers
Cadaveric material has long been used for medical training. Four cadaver preparations are available: formaldehyde based, plastinated, fresh frozen, and soft embalmed.
Formaldehyde is a traditional embalming fluid that denatures proteins and kills bacteria. Cadavers embalmed with formaldehyde are stiff and not amenable to ultrasound examination.
Formaldehyde is a highly toxic and carcinogenic chemical that is recognized as an environmental hazard. The process of plastination was invented by Gunther von Hagens in 1977. Water and lipid tissues are replaced by curable polymers such as silicone and epoxy resin polymers.
Fresh frozen cadavers are unembalmed cadavers available in the United States or imported to other countries. The principal advantage of fresh frozen cadavers is the retention of fine anatomic detail that embalming can destroy. Such cadavers are commonly used on UGRA training courses because sonoanatomy is life-like, and probe handling, needle-probe alignment, and needle tracking and tactile feedback are realistic. The downside is that fresh frozen cadavers decompose quickly and tolerate repeated injection poorly.
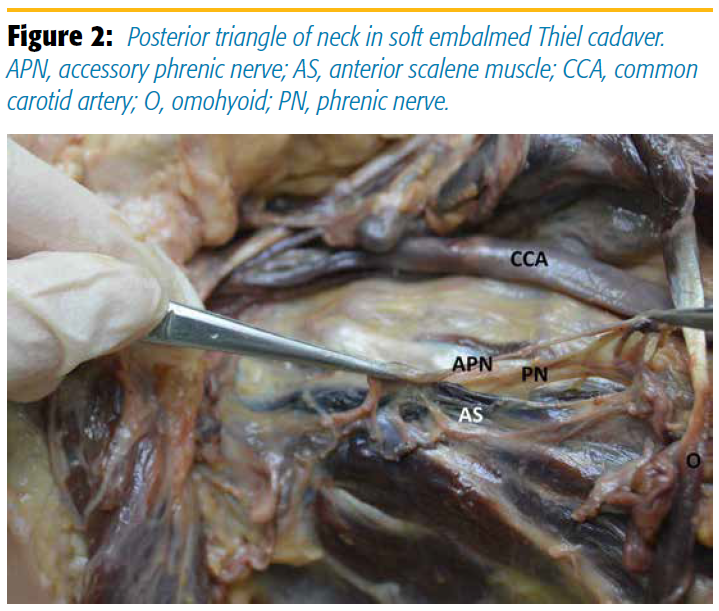
APN, accessory phrenic nerve; AS, anterior scalene muscle; CCA, common carotid artery; O, omohyoid; PN, phrenic nerve
Soft preparation or soft-fix methods have been introduced that offer the acoustic properties of fresh frozen cadavers and the durability of formaldehyde-based cadavers. Many soft embalmed cadavers are prepared with the Thiel method. Cadavers are soaked in large vats containing solutions of boric acid, propylene glycol, and a very small amount of formaldehyde13 for up to six months. The advantage of this technique is that cadavers remain soft and flexible. (Figure 2.) Repeated injection is possible, even at the same site, because they retain elasticity. Circumferential injection of embalming fluid around nerves is accompanied by distention then relaxation of tissues within the same time frame as human injection. Thiel cadavers last as long as three years, but that best histology is maintained for 12 months. Although it is the best available UGRA simulator, the cost of preparation and storage is prohibitive, and only a few medical schools have such cadavers.
Immersive Technology
Augmented reality (AR) and virtual reality (VR) technology, developed from the computer games industry, are changing the way humans learn. AR is “a technology that superimposes a computer-generated image on a user's view of the real world, thus providing a composite view,”14 whereas VR is “the computer-generated simulation of a three-dimensional image or environment that can be interacted with in a seemingly real or physical way by a person using special electronic equipment, such as a helmet with a screen inside or gloves fitted with sensors.”15
Immersive systems provide 3D representations of anatomy and clinical interaction that can be explored individually on a computer screen and learned intuitively, thus reducing cognitive overload. Two examples have been described for UGRA. One used AR to demonstrate tracked ultrasound imaging to help identify vertebral levels; the information was displayed on a live video feed during epidural insertion.16 The other provided anatomical variation of the inguinal region, thus intensifying the immersive experience.17
Immersive technology, in contrast to the previously described models, provides a high-fidelity simulated experience. It meets many of the characteristics of an ideal simulation. However, its disadvantages include high cost, limited accessibility, and the need for ongoing IT support. It is not yet clear what balance of low- and high-fidelity models achieves optimal training.
Conclusion
UGRA simulation models range from the simple to complex, but little research has been conducted to guide teachers. The role of low- and high-fidelity models has yet to be established. The link between simulator performance and clinical performance is not known. The establishment of the ASRA educational research group acknowledges the lack of evidence in this fledgling field and provides a fulcrum for clinicians around the globe to participate in discovering the best way to train residents and ultimately provide a safe environment for patients.
References
- McIndoe AK. Modern anaesthesia training: is it good enough? Br J Anaesth. 2012;109:16–20. https://doi.org/10.1093/bja/aes174
- Munirama S, Zealley K, Schwab A, et al. Trainee anaesthetist diagnosis of intraneural injection-a study comparing B-mode ultrasound with the fusion of B-mode and elastography in the soft embalmed Thiel cadaver model. Br J Anaesth. 2016;117:792–800. https://doi.org/10.1093/bja/aew337
- Barrington MJ, Wong DM, Slater B, Ivanusic JJ, Ovens M. Ultrasound-guided regional anesthesia: how much practice do novices require before achieving competency in ultrasound needle visualization using a cadaver model. Reg Anesth Pain Med. 2012;37:334–339. https://doi.org/10.1097/AAP.0b013e3182475fba
- Ahmed OM, O'Donnell BD, Gallagher AG, Shorten GD. Development of performance and error metrics for ultrasound-guided axillary brachial plexus block. Adv Med Educ Pract. 2017;8:257–263. https://doi.org/10.2147/AMEP.S128963
- Department of Health and Social Care. A framework for technology enhanced learning. 2011. https://www.gov.uk/government/publications/a-framework-for-technology-enhanced-learning. Accessed September 8, 2018.
- Greaves JD. Training time and consultant practice. Br J Anaesth. 2005;95(5):581–583. https://doi.org/10.1093/bja/aei233
- Sultan SF, Shorten G, Iohom G. Simulators for training in ultrasound guided procedures. Med Ultrason. 2013;15(2):125–31.
- CAE Healthcare. Blue phantom ultrasound training medical models. http://www.bluephantom.com/product/Regional-Anesthesia-Ultrasound-Training-Block-Model.aspx?cid=525. Accessed September 8, 2018.
- Baranauskas MB, Margarido CB, Panossian C, Silva ED, Campanella MA, Kimachi PP. Simulation of ultrasound guided peripheral nerve block: learning curve of CET-SMA/HSL anaesthesiology residents. Rev Bras Anestesiol. 2008;58(2):106–111.
- Mooney JJ, Sarwani N, Coleman ML, Fotos JS. Evaluation of three-dimensional printed materials for simulation by computed tomography and ultrasound imaging. Simul Healthc. 2017;12(3):182–188. https://doi.org/10.1097/SIH.0000000000000217
- Niazi AU, Ramlogan R, Prasad A, Chan VW. A new simulation model for ultrasound-aided regional anesthesia. Reg Anesth Pain Med. 2010: 35: 320–321. https://doi.org/10.1097/AAP.0b013e3181df226b
- Xu D, Abbas S, Chan VWS. Ultrasound phantom for hands-on practice. Reg Anesth Pain Med. 2005;30(6):593–594. https://doi.org/10.1016/j.rapm.2005.08.007
- Munirama S, Eisma R, Columb M, Corner GA, McLeod GA. Physical properties and functional alignment of soft-embalmed Thiel human cadaver when used as a simulator for ultrasound-guided regional anaesthesia. Br J Anaesth. 2016;116:699–707. https://doi.org/10.1093/bja/aev548
- Augmented reality. Oxford English Dictionary. https://en.oxforddictionaries.com/definition/augmented_reality. Accessed September 8, 2018.
- Virtual reality. Oxford English Dictionary. https://en.oxforddictionaries.com/definition/virtual_reality. Accessed September 8, 2018.
- Ashab HA, Lessoway VA, Khallaghi S, Cheng A, Rohling R, Abolmaesumi P. AREA: an augmented reality system for epidural anaesthesia. Conf Proc IEEE Eng Med Biol Soc. 2012:2659–2663. https://doi.org/10.1109/EMBC.2012.6346511https://doi.org/10.1109/EMBC.2012.6346511
- Grottke O, Ntouba A, Ullrich S, et al. Virtual reality-based simulator for training in regional anaesthesia. Br J Anaesth. 2009;103:594–600. https://doi.org/10.1093/bja/aep224x
Leave a commentOrder by
Newest on top Oldest on top