Facial Pain Medical and Surgical Approaches: A Problem-Based Learning Discussion
A 60-year-old female with a history of migraine headaches is referred to the clinic for paroxysmal lancinating left facial pain which started approximately three months prior. At that time she was hospitalized for the workup of recurring TIAs (transient ischemic attacks) which was largely inconclusive. While in the hospital, she started experiencing painful episodes characterized by short ‘electric-like’ jolts of pain, referred to the left face around the nose. The episodes were paroxysmal and initially, they were only happening a couple of times a day, which prompted her to believe they were related to her chronic migraine. In the weeks following her discharge from the hospital, these episodes became more frequent and painful, lasting up to one to two minutes each time, prompting her to seek medical attention.
Upon further investigation, she reports that during these episodes her pain recurs in the same distribution every time, in a well-circumscribed area between her left eye and her nose, and goes deeply to the inside of her mouth and her gum line. The pain can be very intense reaching up to 10/10 on the VAS scale, and is described as electric, throbbing, and at times incapacitating. Her pain can be triggered by cold air or liquids coming in contact with her face, and she avoids seeing her dentist and brushing her teeth regularly for fear of precipitating her symptoms. She reports that exerting pressure on her face using her fist during the episodes can reduce the pain. She works as a customer service representative in a local retail hardware store, and she is worried she will be forced to quit her job, as she feels unsafe climbing ladders and operating machinery during these episodes. She was started on lamotrigine during her hospital admission and she currently takes Lamotrigine 150mg three times a day, together with carbamazepine 100 mg twice a day. Although she is pain-free in between episodes and has noted an overall modest improvement in the frequency of these painful episodes, they remain disabling and she is looking for alternative treatment options.
On exam, cranial nerves are intact bilaterally, there is dysesthesia without allodynia in a thin strip of skin between her eye and the nose on the left side. Her strength and sensation are normal, and reflexes are brisk but symmetric with negative clonus, negative Babinski, and Hoffman. A baseline complete blood count and comprehensive metabolic profile were otherwise unremarkable.
Before this appointment, she underwent a brain MRI with contrast which was ordered with time-of-flight magnetic resonance angiography (MRA), three-dimensional reconstruction, and constructive interference in steady state (CISS) sequences to rule out a possible neurovascular compression.
The MRI demonstrated neurovascular contact of the trigeminal nerve root with the superior cerebellar artery (SCA) at its entry into the brainstem (root entry zone), and she was evaluated by Neurosurgery, who scheduled her for left microvascular decompression surgery (MVD).
Questions
1. What are the diagnostic criteria for trigeminal neuralgia (TN) according to the International Headache Society? Discuss the classification in classical, secondary, and idiopathic with respective frequency in the US population.
According to the International Headache Society's International Classification of Headache Disorders (ICHD 3), TN is defined as “recurrent paroxysms of unilateral facial pain localized to the distribution(s) of one or more trigeminal nerves, precipitated by innocuous stimuli within the affected distribution, and not accounted for by another ICHD 3 diagnosis.1 Current guidelines describe three subsets of TN: classical, secondary, and idiopathic. The incidence of trigeminal neuralgia in the United States is 4.3 per 100,000.2
In classical TN, the cause is attributed to neurovascular compression with atrophy or displacement of the nerve root.3 Atrophic changes include, but are not limited to demyelination, neurovascular compromise, and neuronal loss. Neurovascular compression typically occurs at the entry zone of the root. These changes can be viewed by MRI (by measuring volume and cross-sectional area) or during surgical compression of the nerve root as a diagnostic means. The pain distribution of classical TN is usually in V2 or V3 and is more often unilateral. Patients may report a “pre-TN” episode in which they experience continuous pain prior to a paroxysmal presentation. However, between paroxysmal TN episodes, patients are usually asymptomatic. Other patients may experience a milder background pain between paroxysms. Suggested mechanisms for this continuous pain include peripheral or central nerve sensitization.
Most literature attributes secondary TN to multiple sclerosis (MS), cerebellopontine angle tumors, arteriovenous (AV) malformations, other lesions within the distribution of CN V, or any underlying disease that is attributable to neuralgia. MRI is typically the best diagnostic approach for secondary TN, but other tests such as neurophysiological recordings of evoked potentials or trigeminal reflexes may be performed. In MS patients, plaques at the entry zone of the CN V nerve root or pons will lead to TN; however, TN itself is a rare presentation in patients with MS, accounting for 1-2% of the patient population.
Idiopathic TN is identified without a specific abnormality that fulfills the diagnostic criteria of classic TN from MRI or electrophysiological studies. For example, contact between vessels and CN V nerve roots without morphological changes to the nerve root itself may still be present in otherwise symptomatic patients, ruling out classic TN and therefore suggesting an idiopathic cause.
2. What are the clinical features of classical TN (pain location, duration, characteristics)?
The ICHD 3 characterizes the duration of classical TN pain as lasting from a fraction of a second to two minutes. The duration may be prolonged in chronic TN, with a minority of patients reporting pain lasting longer than two minutes most of the time.1
The location, as mentioned previously, is typically unilateral facial pain in the distribution(s) of the trigeminal nerve, and may radiate within the same distribution but not beyond. The location of the pain is more often lateralized to the right over the left, but a bilateral symptom presentation is rare; oftentimes, laterality may switch in different paroxysms. This condition is more commonly seen in women than men.4 Patients report severe, unbearable pain during these spells that may be exacerbated over time. As mentioned previously, while inter-paroxysmal events are usually asymptomatic, a small subset of patients experience a continuous background pain between paroxysms. Alongside the typical severe pain experienced by patients, autonomic symptoms and sensory abnormalities can be associated with TN. Current literature describes the most frequent autonomic symptom as conjunctival tearing or injection; hyperesthesia was the most commonly reported sensory abnormality; and most patients self-reported numbness and/or prickling in the distribution of CN V. Trigger factors that are most often mentioned by patients include chewing, touching, brushing teeth, eating, talking, and cold wind, amongst others; however, triggers may vary between patients. Patients may also report associated headaches or migraine without aura.5
3. What are the typical imaging (structural, DTI) and neurophysiology (trigeminal reflexes) findings in TN?
CN V is the largest cranial nerve and it has a mixed sensory and motor function. In the brainstem, there are three sensory and one motor trigeminal nuclei.6 The cisternal segment of CN V arises from the anterolateral aspect of the pons and consists of a large sensory root comprising the main bulk of the nerve which travels towards Meckel’s cave. Classic TN is most commonly secondary to neurovascular conflict (80-90%), which refers to symptomatic compression of the cisternal segment of CN V by an artery or less commonly a vein. Neurovascular conflict can range from simple contact to severe compression, displacement, and nerve volume loss. The most common offending vessel is the superior cerebellar artery followed by the anterior and inferior cerebellar artery. Aside from the cisternal segment, we can also identify a brainstorm segment which houses the central component of the trigeminal nerve comprising motor and sensory nuclei, a cavernous segment that is mainly defined by the ophthalmic and maxillary divisions of the sensory root, which continues further anteriorly within the lateral wall of the cavernous sinus, and finally the peripheral segments which are the actual V1-3 branches. CN V can be injured in any of these segments.6
MR imaging should include T2W fast spin-echo (FSE) images of the brainstem and upper cervical cord to evaluate the nuclei and fascicular segment of the nerve, pre- and post-gadolinium thin section spin-echo (SE) T1W images in the axial and coronal planes in case a tumor (metastasis or primary CNS) is suspected, and 3D heavily T2-weighted gradient echo sequence (CISS, DRIVE, CE-FAST) for evaluation of the cisternal, cavernous segments, and Meckel's cave. High-resolution TOF (3D FISP or FLASH) and contrast-enhanced MRA are routinely used to evaluate for neurovascular conflicts and pre-surgical planning. CT is not routinely used for pre-operative planning as most centers rely only on high-definition MRI imaging for navigation in MVD surgery. Although the trigeminal nerve can be lesioned in different segments, trigeminal neuralgia is a specific pathologic entity, thought to result from an insult at the root entry zone (REZ), and therefore special attention should be given to this particular anatomic region when imaging the patient.7 The notion that CNS myelin is primarily disrupted at the REZ of TN patients is consistent with peripheral myelin being significantly more resistant to compression and damage. Therefore, the REZ is likely a key region in TN pathophysiology.8
Of note, in patients with ‘true’ trigeminal neuralgia, electrophysiologic studies such as electromyography (EMG) and nerve conduction studies, including blink reflexes should be normal.9 Abnormal trigeminal reflexes are useful indicators to distinguish secondary TN (due to tumor, arteriovenous malformation or multiple sclerosis) from classical and idiopathic TN, with a sensitivity of 80–93% and a specificity of 91–96%.
More recently advanced structural MR imaging methods like DTI (diffusion tensor imaging) have been implemented in the research setting to attempt to stratify patients with TN based on the entity of neurovascular compromise (NVC). DTI provides a noninvasive means for examining trigeminal nerve microstructure in vivo. DTI involves fitting a tensor, which is an ellipsoid-shaped mathematical model, at each brain voxel of a diffusion MR scan. The tensor is characterized by three orthogonal eigenvectors and their associated eigenvalues (λ1, λ2, λ3). In general, the shape of the tensor carries information about the three-dimensional character of the water molecules’ diffusion. Several measures of tissue microstructure can be derived from these eigenvalues, the most widely used being fractional anisotropy (FA).10 FA was lower in the affected REZ of TN patients with NVC, consistent with there being disrupted nerve organization at this location.11 This type of imaging requires specialized imaging processing methods which are usually limited to academic centers.12
In cases where diabetic amyotrophy is suspected, patients can be treated symptomatically as above, with management of hyperglycemia and pain management with options including NSAIDs and amitriptyline.11 Some studies have shown that early intervention with high-dose steroids can lead to more favorable outcomes in these patients, but there has yet been no conclusive evidence supporting use of immunomodulators in patients with diabetic amyotrophy.11
4. What are the current guidelines for the medical management of TN and what are the long term outcomes?
First line pharmacologic therapy for TN is with carbamazepine or oxcarbazepine. A double-blinded study evaluating 24 patients with trigeminal neuralgia showed an initial response with pain reduction in 24 - 72 hours of starting the medication. 70% of the patients saw a complete or good response to the carbamazepine.13 Usual starting dose for Carbamazepine is 100-200mg twice daily, up to 1,800mg per day. This can be increased gradually over weeks by increments of 200mg until pain relief is obtained. Side effects include drowsiness, double vision, dizziness and nausea, and may limit its utility. Alternatively, Oxcarbazepine is typically better tolerated and can be used with a starting dose of 300mg twice daily. Daily dosage can be increased by 300mg every third day to a maximum daily dose of 2,700mg. A retrospective study found that 98% of patients on carbamazepine and 94% on oxcarbazepine had good initial response; however, adverse events were reported in 27% and 18% of patients on Carbamazepine and Oxcarbazepine respectively.14 Gabapentin may be an effective monotherapy, with a meta-analysis demonstrating similar pain relief effects to carbamazepine with a better side-effect profile (26% vs 48% respectively).15 Initial dose is 300mg three times per day, and can be increased by 300mg every third day, up to 3,600mg. Side effects of gabapentin include somnolence, fatigue, vertigo and nausea. There is some weak evidence to suggest Lamotrigine can be considered for refractory pain control; however, treatment dose is 400mg daily, and the prolonged titration period is a limiting factor.16 Typically, lamotrigine is started at 25mg daily for two weeks, followed by 50mg daily for two weeks, and increases of 50mg every one to two weeks thereafter. Intranasal lidocaine can be considered as an acute rescue therapy. A study looking at 152 patients using lidocaine 2.4% spray intranasally saw a 50% or more reduction in pain in 77.6% and 70.4% of patients at 15 and 30 minutes, respectively.17 Other alternative treatments include, baclofen, topiramate, levetiracetam, and tizanidine; however, the evidence behind their use is limited.
5. What is percutaneous rhizotomy for TN, and what are the outcomes?
In patients who are refractory to maximal medical treatment or who are not candidates for surgery (MVD), rhizotomy is a well-established technique for relieving TN pain that involves controlled lesioning of the nerve fibers in the trigeminal ganglion. Percutaneous rhizotomy is performed mainly using glycerol (GR), radiofrequency thermocoagulation (RF), and balloon compression (BC). The general procedure is performed in the operating room and consists of targeting the Gasser ganglion using a 3.5-inch needle by starting at the patient’s cheek region (usually 25 mm lateral to the angle of the mouth) and aiming to pass through the foramen ovale under fluoroscopy guidance, sometimes with the aid of neuronavigation. During this phase, there is a possibility to injure the neighboring ICA in the C2-C4 tract. The needle is advanced, carefully not to enter the buccal mucosa, through the foramen to reach the retro-gasserian space (Meckel’s cave), which can be confirmed by CSF flow from the trigeminal cistern and by seeing the tip of the needle in lateral fluoroscopy intersecting the clivus about 5-10mm below the sellar floor [18]. At that point, the inside cannula is retracted and a stimulating electrode is placed in the ganglion. The electrode depth determines the division of the trigeminal nerve to be stimulated, with the V3 branch being closer to the clivus compared to V1. There is great care not to extend beyond 10mm from the clivus to reduce the risk of injuring CN IV and VI. Once at target, stimulation-induced paresthesia in the corresponding branch distribution serves as confirmatory placement. At this point, the provider may decide to inject 1cc of glycerol in GR, or increase the current going through the electrode to create an ablation in RF. In the case of BC, a small balloon is inflated with about 1 mL of water-soluble contrast and kept under pressure for about five minutes to lesion the ganglion; according to some authors, this method is especially suited to treat V1 TN.19 Potential side effects of lesioning include anesthesia dolorosa, hypoesthesia, or paresthesia, reduced corneal reflex, or keratitis (especially after V1 ablation), and mastication weakness.
Despite being all associated with pain relief, with the presence of one- and three-year outcomes showing up to 61 and 50% pain reduction, respectively, there are different side effects profiles and indications for each technique.20 In general, RF appeared more likely to provide immediate pain relief but with higher risk of anesthesia in the trigeminal distribution. BC was associated with a slightly higher risk of mastication weakness and diplopia due to CN IV or CN V palsy. Ultimately, there are no RTC comparing the three different approaches so the choice may vary depending on the experience of each center. Pain recurrence after the procedure is described as up to 30% in several series with long term (10+ years) follow up.21
6. What are the surgical treatment options for TN, and what are the outcomes?
Microvascular decompression (MVD) is a major neurosurgical procedure which requires access to the posterior cranial fossa and careful dissection to identify the trigeminal nerve and surrounding structures. The usual indication is a patient with TN with brain MRI showing CN V compression by a neurovascular bundle. Typically, a small (two to four inches) craniotomy flap is made in the lateral posterior fossa, below the confluence of the sinuses. After the durotomy and opening of the arachnoid to allow CSF egress with cerebellar decompressionthe V nerve is identified in the cerebellar-pontine angle and followed to the root entry zone to search for compression.22 The most involved vessel is the superior cerebellar artery but more than one vessel may be involved, arachnoid adhesions may be present, and vertebrobasilar ectasia is sometimes found as well (often associated with hemifacial spasm). At that point, the nerve is decompressed with a variety of methods: interposed pads, Teflon, or no-contact decompression using slings. After closure, the patients may be immediately pain free and then slowly weaned off their medications.
Gamma knife (GK) radiosurgery is limited to patients who are either not candidates to MVD surgery or who do not have an obvious anatomical lesion intersecting their V cranial nerve. GK involves targeting with stereotactic accuracy (sub-millimeter precision) the root entry zone of the V nerve: in general, around 201 gamma beams coming from the cobalt 60 sources of the Gamma Knife machine intersect within 0.3 mm from the source and it is possible to align the selected target point with the focal point within a mechanical accuracy of 0.5 mm.23 The patient’s head is first stabilized in a stereotactic frame with pins secured to the skull. After this step, a high definition 0.75mm slice thickness computerized tomography (CT) of the head is performed and the images uploaded to the GK planning station to be co-registered with the patient’s MRI head. At this point, after selecting the region of interest close to the root entry zone, a radiation oncology technician aids in selecting the number of isocenters (points in space relative to the treatment machine about which various components of the gamma source rotate) and collimators for the final treatment. A typical treatment for TN delivers about 80 Grays of energy using a single isocenter in a time ranging from 28 to 69 minutes.24 Differently from MVD, no incisions are made and the pain relief is not instantaneous as you need to wait for the tissue to degenerate following the radiation exposure which takes several weeks.
MVD is the method of choice if a relatively young patient is suffering from TN and has an obvious vascular compression. Several meta-analyses have demonstrated the superiority of long term (more than five years after the procedure) pain relief after MVD. For example, in a cohort of 49 patients treated with GK and 20 by MVD, there was no significant difference between the two groups with respect to initial pain relief (100% MVD, 84% GK; p = 0.055), neither was there a significant difference in pain recurrence (39% GKRS, 20% MVD; p = 0.133.25 At last follow-up, 85% of patients who underwent MVD had total pain relief (BNI scale I) compared to only 45% of GKRS patients (p = 0.002).
7. What is the difference between TN and atypical facial pain and neuralgia?
TN is defined as recurrent paroxysms of unilateral facial pain in the distribution of one or more of the divisions of the trigeminal nerve. Pain will last fractions of a second to two minutes. It is severe in quality and described as shock-like, stabbing, or shooting. The pain is precipitated by innocuous stimuli. As in the case above, cold air, liquids, and brushing her teeth are all precipitating stimuli. Postherpetic neuralgia can cause pain similar to that of TN in the distribution of the trigeminal nerve. Postherpetic neuralgia is usually preceded by reactivation of the Varicella Zoster Virus and subsequent vesicular eruptions in a dermatomal pattern. These vesicles will rupture and heal while the acute neuritis may be followed by persistent neuropathic pain in the same distribution.27 In contrast, Persistent Idiopathic Facial Pain (PIFP) is another diagnosis that is on the differential when considering facial pain. PIFP is a persistent, long lasting, and daily pain that will change over time. The pain is poorly localized and does not follow a dermatomal distribution. The pain can be described as burning, dull, aching, or nagging. Pain is worsened during periods of stress.26 The near-continuous nature of both PIFP and Postherpetic neuralgia help to distinguish it from Trigeminal Neuralgia’s paroxysmal presentation.27
8. Can you describe a working diagnostic-therapeutic algorithm for facial pain?
The diagnosis of TN is primarily clinical, with imaging used to evaluate contributing factors. History should include the following:
a. Distribution of the pain (specific dermatome [V2, V3 more common than V1], laterality [TN is typically unilateral])
b. Type of pain (electric shock-like pain, typically one to two seconds)
c. Triggers (zones in the face where light touch causes the pain, typically midline [pathognomonic for TN] and specific actions such as chewing, cold air [described above])4,14
d. Associated symptoms (V1 involvement can produce mild autonomic symptoms such as lacrimation, rhinorrhea, and conjunctival injection)4
Physical and neurologic exams are typically normal except for potential trigger zones, and findings such as sensory loss, weakness, or loss of corneal reflex, should trigger further work up for secondary causes. In clinically suspected TN, a high-resolution MRI of the brain with and without contrast is recommended to identify vascular compression and rule out structural brain lesions, such as MS, and cerebellopontine angle tumor. If a secondary cause of TN is identified, appropriate treatment should be initiated. Initial treatment in patients with classic TN is pharmacologic with carbamazepine, or oxcarbazepine, with the latter being better tolerated. Second line medications include baclofen, gabapentinoids, and lamotrigine.28 In patients who are older, refractory to therapy, or who cannot tolerate medication, botox injections, rhizotomy, peripheral neurectomy and nerve block, and gamma knife radiosurgery can be considered. In younger patients refractory to medical therapy, microvascular decompression can be performed if vascular compression is identified as the cause of TN. Differential diagnosis for trigeminal neuralgia includes post herpetic neuralgia, dental pain, short-lasting unilateral neuralgiform headache attacks (SUNA), short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT), trigeminal neuropathy, and temporomandibular joint syndrome (Figure 1 & 2).29
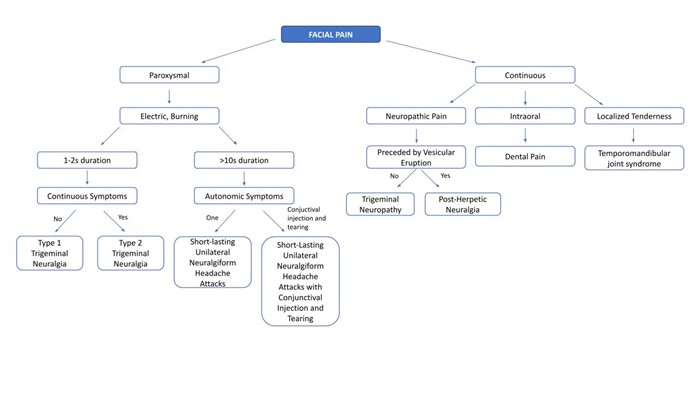 Figure 1: Differential diagnosis of facial pain starting from clinical presentation.
Figure 1: Differential diagnosis of facial pain starting from clinical presentation.
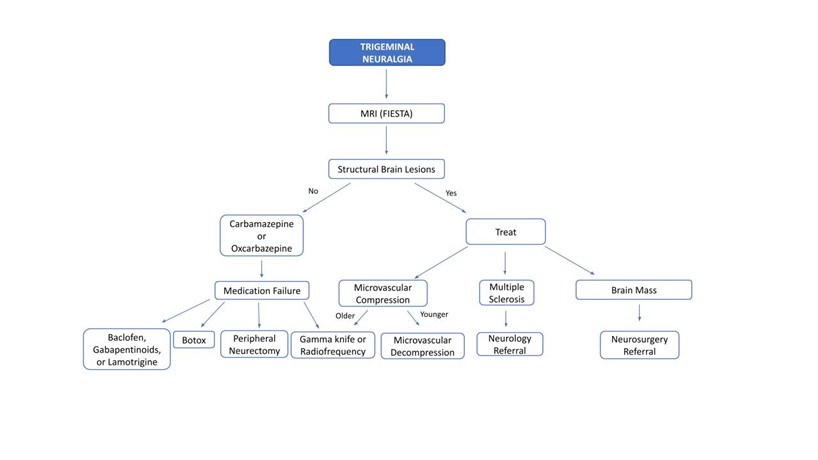 Figure 2: TN diagnostic-therapeutic practical algorithm.
Figure 2: TN diagnostic-therapeutic practical algorithm.
References
1. Feldman EL, Callaghan BC, Pop-Busui R, et al. Nat Rev Dis Primers 2019;5(41). https://doi.org/10.1038/s41572-019-0092-1
2. Tracy JA, Dyck PJ. The spectrum of diabetic neuropathies. Phys Med Rehabil Clin N A 2008;19(1):1-26. https://doi.org/10.1016/j.pmr.2007.10.010
3. Bodman MA, Varacallo M. Peripheral diabetic neuropathy. StatPearls [Internet]. 2023 https://www.ncbi.nlm.nih.gov/books/NBK442009. Published September 4, 2023. Accessed November 13, 2023.
4. Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40(1):136-54. https://doi.org/10.2337/dc16-2042
5. Pop-Busui R, Ang L, Boulton AJM, et al. Diagnosis and Treatment of Painful Diabetic Peripheral Neuropathy. Arlington, VA: American Diabetes Association; 2022.
6. Lindsay TJ, Rodgers BC, Savath V, et al. Treating diabetic peripheral neuropathic pain. Am Fam Physician 2010;82(2):151-58.
7. Bril V, England JD, Franklin GM, et al. Evidence‐based guideline: treatment of painful diabetic neuropathy—report of the American Association of Neuromuscular and Electrodiagnostic Medicine, the American Academy of Neurology, and the American Academy of Physical Medicine & Rehabilitation. Muscle Nerve 2011;43(6):910-17. https://doi.org/10.1212/WNL.0b013e3182166ebe
8. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14(2):162-73. https://doi.org/10.1016/S1474-4422(14)70251-0
9. Price R, Smith D, Franklin G, et al. Oral and topical treatment of painful diabetic polyneuropathy: practice guideline update summary: report of the AAN Guideline Subcommittee. Neurology 2022;98(1);31-43. https://doi.org/10.1212/WNL.0000000000013038
10. Tesfaye S, Sloan G, Petrie J, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet 2022;400(10353): 680-90. https://doi.org/10.1016/S0140-6736(22)01472-6
11. Diaz LA, Gupta V. Diabetic amyotrophy. StatPearls [Internet]. 2023 https://www.ncbi.nlm.nih.gov/books/NBK560491. Published August 14, 2023. Accessed November 13, 2023.
12. Wang EJ, Berninger LE, Komargodski O, et al. Painful diabetic neuropathy-spinal cord stimulation, peripheral nerve stimulation, transcutaneous electrical nerve stimulation, and scrambler therapy: A narrative review. Pain Physician 2022;25(8):E1163-73.
13. Perkins BA, Ngo M, Bril V. Symmetry of nerve conduction studies in different stages of diabetic polyneuropathy. Muscle Nerve 2002;25(2):212-17. https://doi.org/10.1002/mus.10044
14. Dyck, PJ, Giannini C. Pathologic alterations in the diabetic neuropathies of humans: a review. Journal of Neuropathology & Experimental Neurology 1996;55(12),1181-93. https://doi.org/10.1097/00005072-199612000-00001
15. Kayser-Gatchalian MC, Neundörfer B. Sural nerve conduction in mild polyneuropathy. J Neurol 1984;231(3):122-25. https://doi.org/10.1007/BF00313678
16. Johnson EW, Pease, WS. Practical Electromyography. Baltimore, MD: Williams & Wilkins; 1988.
17. Perkins BA, Olaleye D, Bril V. Carpal tunnel syndrome in patients with diabetic polyneuropathy. Diabetes Care 2002;25(3):565-69. https://doi.org/10.2337/diacare.25.3.565
18. Miscio G, Guastamacchia G, Brunani A, et al. (2005). Obesity and peripheral neuropathy risk: a dangerous liaison. J Peripher Nerv Syst 2005;10(4):354-58. https://doi.org/10.1111/j.1085-9489.2005.00047.x
19. Ziegler D, Rathmann W, Dickhaus T, et al. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med 2009;10(2):393-400. https://doi.org/10.1111/j.1526-4637.2008.00555.x
20. American Diabetes Association. Postprandial blood glucose. Clin Diabetes 2001;19(3):127-130. https://doi.org/10.2337/diaclin.19.3.127
21. Tesfaye S, Chaturvedi N, Eaton SE. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352(4):341-50. https://doi.org/10.1056/NEJMoa032782
22. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep 2014;14(9):528. https://doi.org/10.1007/s11892-014-0528-7
23. Herrmann D, Griffin J, Hauer P, et al. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology 1999;53(8): 1634-40. https://doi.org/10.1212/wnl.53.8.1634
24. Tobin K, Giuliani MJ, Lacomis D. Comparison of different modalities for detection of small fiber neuropathy. Clin Neurophysiol 1999;110(11):1909-12. https://doi.org/10.1016/s1388-2457(99)00164-9
25. Van Maurik JFMM, van Hal M, van Eijk RPA, et al. Value of surgical decompression of compressed nerves in the lower extremity in patients with painful diabetic neuropathy: a randomized controlled trial. Plast Reconstr Surg 2014;134(2):325-32. https://doi.org/10.1097/PRS.0000000000000369
26. Xu L, Sun Z, Casserly E, et al. Advances in interventional therapies for painful diabetic neuropathy: a systematic review. Anesth Analg 2022;134(6):1215-28. https://doi.org/10.1213/ANE.0000000000005860
27. Feller L, Khammissa R, Fourie J, et al.
Postherpetic neuralgia and trigeminal neuralgia. Pain Res Treat 2017;2017:1681765. https://doi.org/10.1155/2017/1681765
28. Shankar Kikkeri N, Nagalli S. Trigeminal
Neuralgia.StatPearls [Internet]. 2023. https://www.ncbi.nlm.nih.gov/books/NBK442009. Accessed November 13, 2023.
29. VanderPluym J, Richer L. Tic versus TAC:
differentiating the neuralgias (trigeminal neuralgia) from the cephalalgias
(SUNCT and SUNA). Curr Pain Headache Rep
2015;19:1-6. https://doi.org/10.1007/s11916-014-0473-9