Recommendations on Chronic Pain Practice during the COVID-19 Pandemic
A Joint Statement by American Society of Regional Anesthesia and Pain Medicine (ASRA) and European Society of Regional Anesthesia and Pain Therapy (ESRA)
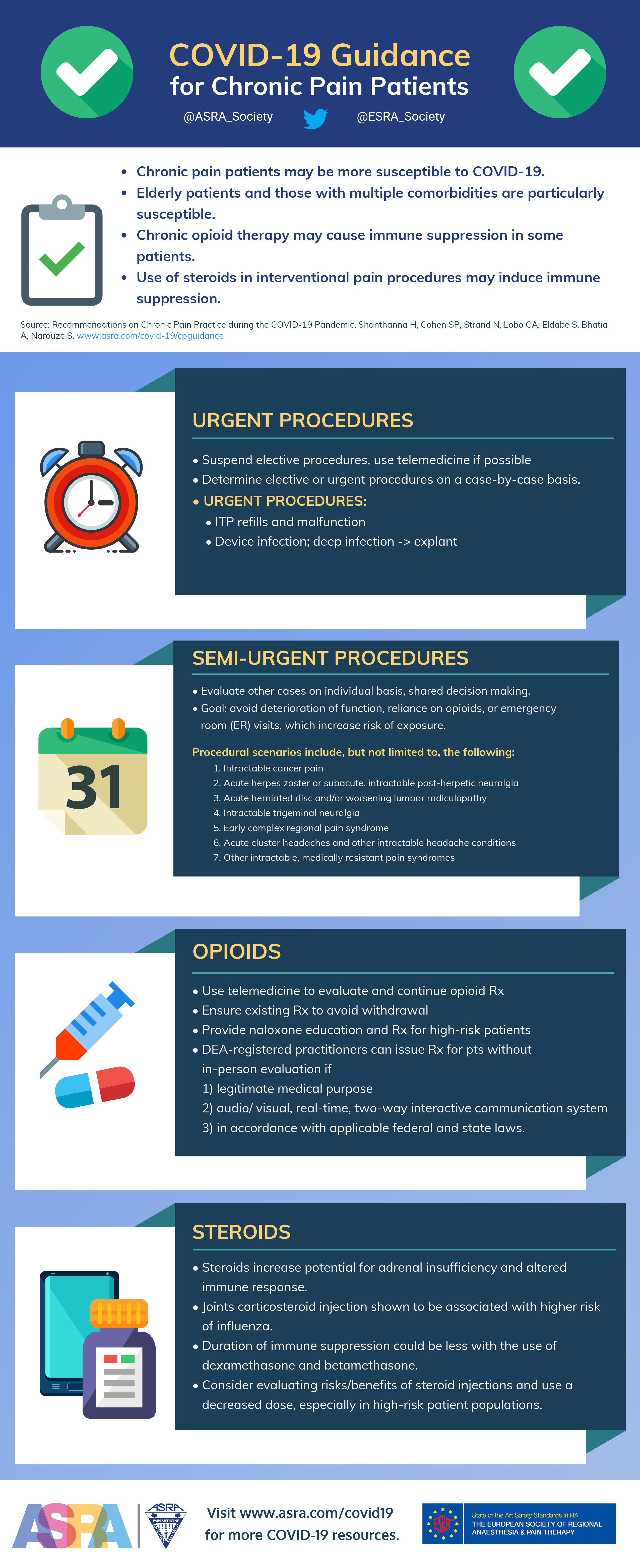
Authors: Harsha Shanthanna, MBBS, MD, MSc, FRCPC; Steven P. Cohen, MD; Natalie Strand, MD; Clara Lobo, MD; Sam Eldabe, MBBS, FRCA; Anuj Bhatia, MBBS, MD, FRCA, FRCPC; Samer Narouze, MD, PhD
Note: As the situation continues to rapidly evolve, the following represents current guidance based on the best available evidence and expert opinion. This document was last updated on March 27, 2020.
General Background
Novel coronovirus-2019 (initially referred as 2019-nCoV) is a human b-coronavirus that has been renamed as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). From the observation of the initial 1099 patients with confirmed COVID-19 infection, 6.1% patients had severe infection (ICU admission, invasive mechanical ventilated, or death). Common symptoms were fever (43.8% on admission and 88.7% during hospitalization) and cough (67.8%). The median incubation period was 4 days (interquartile range, 2 to 7).1 Because of the highly infectious nature of the disease, the risk of transmission is high. The mortality rate seems to be higher than previous estimates noted from China; mortality rates would be 5.6% (95% CI 5.4–5.8) for China and 15.2% (95% CI 12.5–17.9) outside of China.2 These numbers are likely to change over time as COVID-19 testing increases, especially in asymptomatic patients, leading to more positive cases and a drop in fatality rates. Mortality and morbidity can be caused by acute respiratory distress syndrome, arrhythmia, shock, acute kidney injury, acute cardiac injury, liver dysfunction, and secondary infection.1,3
General Considerations in Chronic Pain Patients
- Susceptibility of chronic pain patients could be higher as many are elderly with multiple comorbidities and potential immune suppression.4,5
- Significant immune changes occur in a patient with COVID-19 disease.3,6 Chronic pain exerts complex effects on the immune system, including immunosuppression in some individuals.7
- Chronic opioid therapy may cause immune suppression in some patients, and individual opioids differ in their potential.8,9
- Use of steroids in interventional pain procedures may induce immune suppression. Intraarticular corticosteroid injections have been associated with higher influenza risk.10
General Recommendations
1. Any elective, in-person patient visits or meetings have to be suspended.
- Whenever possible, telemedicine should be considered. Although most jurisdictions have relaxed the administrative restrictions around the use of telemedicine, practitioners must ensure that the interface used satisfies the rules and regulations of their place of practice.
2. No elective pain procedures, except specific semi-urgent procedures, should be performed.
- Across most parts of the world, all elective surgeries and procedures have been postponed or cancelled. Reasons include decreasing the exposure of patients and healthcare providers and conserving resources already strained by diminished production capacity and travel and shipping restrictions during this public health crisis.11
- Categorizing pain procedures as elective, urgent, and emergent is – in many cases – subjective. For example, not performing or postponing a procedure may lead to significant morbidity and other adverse sequelae. In chronic pain patients, withholding pain management services could lead to inability to work, anxiety and depression, and reliance on opioid therapy. The American College of Surgeons provides some direction in this regard, noting that both medical and logistical contexts must be considered on a case-by-case basis.12
- Although most chronic pain interventions fall under the elective category, there are some situations that fall into the urgent or emergent categories. Below are examples of such procedures along with recommendations on management.
Scenarios of “urgent” pain patient procedures during the COVID-19 pandemic
Intrathecal pump (ITP) refills and malfunction
- ITP refills necessitate in-person meeting and evaluation. See below for information regarding performing procedures and precautions.
- Use telemedicine as much as possible to resolve or sort out issues.
- End-of-life ITP battery requires urgent replacement to avoid withdrawal symptoms.
- Avoid insertion of any new ITPs, except for highly selected cancer pain cases where the benefit is considered to outweigh the risk.
- Following careful discussion with the patient, consider the risk/benefit of using higher drug concentrations for the period of the pandemic in order to reduce ITP refill visits.
Neurostimulator infection and malfunction
- If an implant infection is suspected, an in-person evaluation may be necessary. Depending on whether the infection is superficial or deep, device explant may be warranted and should be performed as soon as possible.13
- Use telemedicine as much as possible to resolve and sort out issues.
- Avoid any new trials or implants.
Scenarios of “semi-urgent” pain patient procedures during the COVID-19 pandemic
- Other cases should be evaluated on an individual basis, with shared decision making. The goal is to avoid deterioration of function, reliance on opioids, or emergency room (ER) visits that increase risk of exposure.
- Use telemedicine to evaluate the patient, triage the urgency, and make suitable arrangements for treatment. This will minimize delay and prevent unnecessary visits.
- Such procedural scenarios may include, but are not limited to, the following:
- Intractable cancer pain
- Acute herpes zoster or subacute, intractable post-herpetic neuralgia
- Acute herniated disc and/or worsening lumbar radiculopathy
- Intractable trigeminal neuralgia
- Early complex regional pain syndrome
- Acute cluster headaches and other intractable headache conditions
- Other intractable medically resistant pain syndromes (should be reviewed on a case-by-case basis)
Opioids and COVID-19
Significant immune changes occur in patients with COVID-19 disease.3,6 Most patients have normal or decreased white blood cell counts and lymphocytopenia. The potential for thrombocytopenia exists in severe cases.1,14 Opioids are recognized as causing immune suppression, and individual opioids differ in their potential.8,9 Although some have observed buprenorphine to have less effect on animals’ immune systems,9,15 it is not clear if this is consistently observed in humans.8 Some suggest that there may be beneficial immune effects by opioids as well.16 Patients with COVID-19 who are receiving opioids can be more susceptible to respiratory depression, and the absorption of fentanyl during transdermal administration (fentanyl patch) may increase with fever.
- We do not recommend any changes to ongoing opioid treatment regimens in the absence of documented changes in pain and/or function.
- We do recommend careful monitoring of patients on transdermal opioids, as the rate of absorption with high fever can be unpredictable.
Opioid prescriptions and telemedicine
Changes to opioid prescriptions should be made only after careful evaluation of ongoing treatment, which ideally includes an in-person history and physical exam. However, considering the nature of the current COVID-19 health emergency, it is appropriate to make changes and/or continue prescriptions using telemedicine.
- Use telemedicine to evaluate and continue opioid prescriptions.
- Ensure adherence to the subscribed needs of telemedicine required by your state or country of practice.
- Ensure all patients receive their appropriate prescription of opioids to avoid withdrawal.
- Provide naloxone education and prescription for high-risk patients.
In the United States, the Secretary of Health and Human Services declared a public health emergency on January 31, 2020, under 42 U.S.C. 247d (section 319 of the Public Health Service Act), as set forth in 21 U.S.C. 802(54)(D).17 Based on this edict, any telemedicine allowance under section 802(54)(D) applies to all schedule II-V controlled substances in all areas of the United States. Accordingly, the Drug Enforcement Administration (DEA) notes that DEA-registered practitioners may issue prescriptions for controlled substances to patients for whom they have not conducted an in-person medical evaluation, provided the following conditions are met:18
- The prescription is issued for a legitimate medical purpose by a practitioner acting in the usual course of his/her professional practice
- The telemedicine communication is conducted using an audio-visual, real-time, two-way, interactive communication system
- The practitioner is acting in accordance with applicable Federal and State laws.
Use of Anti-Inflammatories for Chronic Pain
Although a single report suggested that the use of non-steroidal anti-inflammatory drugs may increase the severity of COVID-19 disease19, most health authorities note that the evidence is not definitive.20-22 However, anti-inflammatory drugs may mask early symptoms of the disease such as fever and myalgias.
- We recommend that all patients who have been prescribed or use non-steroidal anti-inflammatory drugs on a regular basis to continue using them.
- We recommend educating patients who are taking anti-inflammatory drugs to promptly report mild fever or new myalgia.
Steroids in Chronic Pain and COVID-19
- Chronic pain patients may be on oral steroids or may have received a recent steroid intervention.
- Patients on steroids have a potential for secondary adrenal insufficiency and altered immune response.23
- It is appropriate to discuss any new therapy that may influence the course of COVID-19 disease with the treating infectious disease physician, as using steroids for all patients as a treatment adjunct in COVID-19 is not recommended.6
- Injections of corticosteroids into joints was shown to be associated with a higher risk of influenza.10
- The duration of immune suppression could be less with the use of dexamethasone and betamethasone.24
- Consider evaluating the risks and benefits of steroid injections, and use a decreased dose, especially in high-risk patient populations. There are many procedures in which steroids are routinely used, wherein the evidence does not support the practice.25
Procedural Precautions and Conduct of Procedure
Any patient who has been determined to need a procedure or an in-person meeting must be screened for the possibility of COVID-19. A history of travel to high-risk areas or countries and the presence of symptoms increase the likelihood of infection, but patients who self-quarantine may become infected and asymptomatic transmission is also possible. Once the community spread of COVID-19 becomes significant, all cases may be presumed to be COVID-19 positive. Local guidelines should be followed when making decisions. Detailed information for protection of patients and health care providers is provided in various websites, including the Centers for Disease Control (https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html) and the European Centre for Disease Prevention and Control (https://www.ecdc.europa.eu/en/all-topics-z/coronavirus/threats-and-outbreaks/covid-19/preparedness-and-response-covid-19).
In a COVID-19 negative or a low-risk patient
- Ensure there is minimal patient movement around the hospital.
- Meet the patient in a clean room with no prior exposure to COVID-19 positive patients.
- Procedures should be carried out by an experienced person.
- Ensure that the needed medications (for ITP refill) and equipment are ready and transported in a fully covered plastic bag. Open the bag with disposable gloves and take out the medications in a clean area.
- These procedures do not lead to aerosol generation; hence contact and droplet precautions must be taken. Ensure an accessible hand-wash area and hand sanitizer are available.
- Utilize surgical mask, eye protection, surgical gown, and double gloves for personnel involved in performing the procedure. The use of N95 or similar powered air-purifying respirator (PAPR) is not necessary.
- The patient should wear a surgical mask to reduce the chance of droplet spread.
- The device-programming equipment – particularly parts that come in contact with the patient such as ultrasound equipment and transducer – should be protected from contamination using plastic covers. Bringing carts or trolleys with drugs and equipment to the procedure room should be discouraged.
- Minimize the number of personnel present during the performance of the procedure, but help should be readily available.
- Routine aseptic technique should be followed. An in-vitro study suggests that COVID-19 virus particles are viable for longer on plastic than cardboard. A change in practice to use sterile paper drapes instead of these transparent plastic ones should be considered if available.26
In a COVID-19 positive or a high-risk patient
- Strictly limit these patients to urgent procedures.
- The procedure or meeting should be performed in an area or procedure room designated for use with COVID-19 patients. This ensures that protective equipment is available and precautions are followed.
- The use of common areas, such as a block room or a holding area, should be avoided as they can lead to contamination.
- These procedures do not lead to aerosol generation; hence contact and droplet precautions must be taken. However, consideration of the use of N95 mask may be made on a case-by-case basis depending on local availability. This depends on the risk of coughing or sneezing by the patient.
- Other precautions are similar to a low-risk patient.
- Post-procedure or after the evaluation, the patient should be monitored in the room until they can be shifted to an isolation room (in hospital) or discharged home with instructions.
- It has been shown that the risk of transmission is highest during the removal of protective gear; hence, appropriate precautions should be taken to remove and discard gloves and masks. It is essential that hands are washed thoroughly after the procedure and that physicians avoid touching their face or other surfaces beforehand.
- The presence of an observer during the donning and doffing procedure is highly recommended. Simulation sessions for the donning and doffing of PPE should be conducted for training staff.
References
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMoa2002032. [Epub ahead of print].
- Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020 Mar 12. pii: S1473-3099(20)30195-X. doi: 10.1016/S1473-3099(20)30195-X. [Epub ahead of print].
- Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020 Mar 13;7(1):11. doi: 10.1186/s40779-020-00240-0.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37-43. doi: 10.1016/S0140-6736(12)60240-2. Epub 2012May 10..
- Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123(2):e273-e83. doi: 10.1016/j.bja.2019.03.023. Epub 2019May 10..
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 Mar 16. pii: S0140-6736(20)30628-0. doi: 10.1016/S0140-6736(20)30628-0. [Epub ahead of print].
- Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267-76.doi: 10.1038/nm.2234. Epub 2010 Oct 14.
- Franchi S, Moschetti G, Amodeo G, Sacerdote P. Do all opioid drugs share the same immunomodulatory properties? a review from animal and human studies. Front Immuno. 2019;10:2914. doi: 10.3389/fimmu.2019.02914.
- Sacerdote P. Opioids and the immune system. Palliat Med. 2006;20 Suppl 1:s9-15.
- Sytsma TT, Greenlund LK, Greenlund LS. Joint corticosteroid injection associated with increased influenza risk. Mayo Clin Proc Innov Qual Outcomes. 2018;2(2):194-8. doi: 10.1016/j.mayocpiqo.2018.01.005.
- Luthi S. Surgeon general advises hospitals to cancel elective surgeries. Politico. 2020. March 14. Available at https://www.politico.com/news/2020/03/14/surgeon-general-elective-surgeries-coronavirus-129405. Accessed March 14, 2020.
- American College of Surgeons. COVID-19: guidance for triage of non-emergent surgical procedures. Available at https://www.facs.org/about-acs/covid-19/information-for-surgeons/triage. Accessed March 17, 2020.
- Deer TR, Provenzano DA, Hanes M, et al. The Neurostimulation Appropriateness Consensus Committee (NACC) recommendations for infection prevention and management. Neuromodulation. 2017;20(1):31-50. doi: 10.1111/ner.12565.
- Lippi G, Plebani M, Michael Henry B. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Act. 2020Mar 13. pii: S0009-8981(20)30124-8. doi: 10.1016/j.cca.2020.03.022. [Epub ahead of print].
- Gomez-Flores R, Weber RJ. Differential effects of buprenorphine and morphine on immune and neuroendocrine functions following acute administration in the rat mesencephalon periaqueductal gray. Immunopharmacology. 2000;48(2):145-56.
- Plein LM, Rittner HL. Opioids and the immune system - friend or foe. Br J Pharmacol. 2018;175(14):2717-25.
- U.S. Department of Health and Human Services. Secretary Azar declares public health emergency for United States for 2019 novel coronavirus. 2020 Jan 31. Available at https://www.hhs.gov/about/news/2020/01/31/secretary-azar-declares-public-health-emergency-us-2019-novel-coronavirus.html. Accessed February 1, 2020.
- Diversion Control Division, Drug Enforcement Administration. COVID-19 information page. Available at www.deadiversion.usdoj.gov/coronavirus.html. Accessed February 17, 2020.
- Day M. COVID-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ 2020 2020;368:m1086. doi: 10.1136/bmj.m1086.
- BMJ Best Practice. Coronavirus disease 2019 (COVID-19) Available at https://bestpractice.bmj.com/topics/en-gb/3000168/treatment-algorithm#referencePop126. Accessed March 17, 2020.
- U.S. Food and Drug Administration. FDA advises patients on use of non-steroidal anti-inflammatory 19. 2020 March 19, 2020. Available at https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-patients-use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19. Accessed March 19, 2020.
- European Medicines Agency. EMA gives advice on the use of non-steroidal anti-inflammatories for COVID-19. 2020 March 13. Available at https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19. Accessed March 18, 2020.
- Liu MM, Reidy AB, Saatee S, Collard CD. Perioperative steroid management: approaches based on current evidence. Anesthesiology. 2017;127(1):166-72. doi: 10.1097/ALN.0000000000001659.
- Friedly JL, Comstock BA, Heagerty PJ, et al. Systemic effects of epidural steroid injections for spinal stenosis. Pain. 2018;159(5):876-83. doi: 10.1097/j.pain.0000000000001158.
- Van Boxem K, Rijsdijk M, Hans G, et al. Safe use of epidural corticosteroid injections: recommendations of the WIP Benelux Work Group. Pain Pract. 2019;19(1):61-92. doi: 10.1111/papr.12709.
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Mar 17. doi: 10.1056/NEJMc2004973. [Epub ahead of print].