Implementing Therapy With Opioids in Cancer Patients
Authors
Meda Raghavendra, MD
Associate Professor of Anesthesiology and Pain Medicine
Loyola University Medical Center
Maywood, IL
Julie Sparlin, MD
Private Practice
Phoenix, AZ
Oscar de Leon-Casasola, MD
Professor of Anesthesiology and Medicine
Vice-Chair for Clinical Affairs
Department of Anesthesiology
University at Buffalo School of Medicine and Biomedical Sciences
Chief, Pain Medicine and Professor of Oncology
Roswell Park Cancer Institute
Buffalo, NY
Revised December 30, 2016
Introduction
Pain is one of the most common, and feared, symptoms associated with cancer.[1] Often, the chief complaint of patients with advanced cancer is pain. As patients live longer, there is an increased need for effective pain control to improve quality of life.[2] Although the lack of diagnostic tests makes it difficult to define the exact prevalence, pain occurs in approximately 25% of patients with newly diagnosed cancer, 33% of patients undergoing treatment, and 75% of patients with advanced disease.
Indeed, an estimated 90% of patients experience at least moderate pain at some point in their illness, and 42% do not receive adequate palliation. Undertreated cancer pain is a particular problem in women, minority ethnic groups, and the elderly.[2] Unrelieved pain denies patients comfort and greatly affects activities, motivation, interactions with families and friends, and overall quality of life.[1]
As the area of palliative care continues to increase, efforts to improve pain control will continue to be an essential element of cancer care.[3] Yet there are a number of barriers to effective pain relief including inadequate assessment by practitioners, underreporting of pain by patients and families, practitioners’ lack of knowledge regarding current treatment, lack of accountability for effectively treating pain, fear of overregulation by government officials, and inadequate reimbursement for pain treatment or excessive administrative demands on healthcare providers.[2] In fact, in the nearly 70 years since regulatory controls were placed on opioid use, it has been difficult to rebuild confidence in the use of opioids as an effective, safe, and humane treatment for cancer pain.
With the therapies currently available to clinicians, up to 99% of cancer pain can be controlled with the use of aggressive multimodal pharmacologic therapy and invasive techniques.[2] In 2010, there is no reason for most cancer patients to be in pain.
Classification of Pain
In 1990, the World Heath Organization (WHO) established guidelines for cancer pain relief and palliative care. According to these guidelines, potent opioids such as morphine were reserved for treatment of the most severe pain.
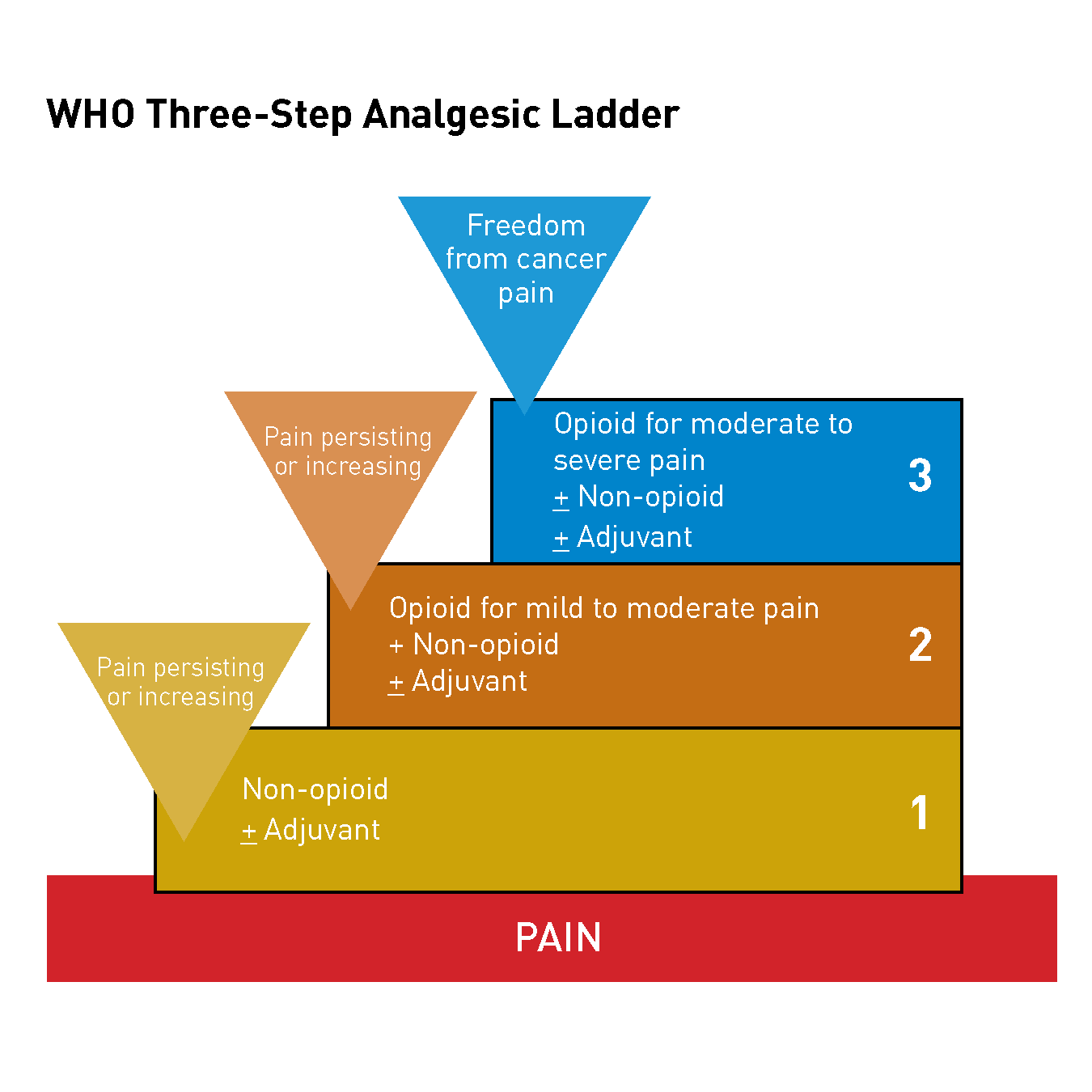
However, the WHO three-step analgesic stepladder approach is no longer the most appropriate strategy for pain management because we now know that the management of cancer pain is complex[1] and not all pain is the same.
Pain associated with cancer results from tumor infiltration of pain-sensitive structures (e.g., nerves, bone, soft tissue). Pain can also result from chemotherapy, radiotherapy, or surgery, and from tumor- or radiation-induced vascular occlusion.[2,4] Therefore, a physiopathologic approach is required to manage patients with cancer pain. This approach includes a thorough patient history, physical examination, and dedicated testing to determine if the pain is visceral, somatic, or neuropathic in nature.[2]
Visceral pain is common in patients with cancer and results from infiltration, compression, distension, or stretching of thoracic and abdominal viscera. Visceral pain usually results from primary tumor growth (e.g., pancreatic cancer) or metastatic tumor growth (e.g., liver metastases).[2,4] Patients describe visceral pain as gnawing, cramping, aching, or sharp.[1] Visceral pain is diffuse and many patients will use their whole hand to tell you where it hurts.
In contrast, somatic pain is well localized;[2,4] patients can tell you where it hurts with one finger. Somatic pain usually occurs in skin, muscle, or bone.[1]
Finally, neuropathic pain results from injury to the peripheral and/or central nervous system. This can be a consequence of tumor compression or infiltration of a peripheral nerve or the spinal cord, or as a result of trauma or chemical injury to a peripheral nerve caused by surgery, radiation, or chemotherapy.[2,4] Neuropathic pain is usually described as sharp, tingling, burning, or shooting.[1] Patients often report the pain as “electric shock” sensations.
Approaches to Pain Management
What outcomes are we trying to achieve with appropriate pain management? Obviously, a primary goal is pain relief or analgesia, but there are three other important “A’s” to consider: activities of daily living (psychologic functioning, sleep), adverse effects, and aberrant drug-related behavior (addiction-related outcomes).
In most patients, adequate pain relief can be achieved initially by administration of either pharmacologic or invasive therapies (e.g., intrathecal therapy, peripheral nerve stimulation, radiofrequency nerve ablation) and by adopting a multidisciplinary approach to supportive care. Management strategies need to incorporate medical options, physical medicine, procedures such as epidurals, and psychologic considerations, as well as complementary approaches like acupuncture (Table 1). Pain management must be approached on all fronts. Cancer patients with pain need the support of numerous specialty groups in order to get the best care we have to offer in the 21st century.
| Approach | Examples |
|---|---|
| Medical | Nonsteroidal anti-inflammatory drugs, opioids, steroids, adjuvants |
| Physical Medicine | Transcutaneous electrical nerve stimulation, heat, physical therapy, ultrasound |
| Procedural | Epidurals, neuromodulation, nerve blocks, trigger point injections, radiation |
| Psychological | Stress management, biofeedback, music |
| Complementary | Acupuncture, massage, herbs |
Pharmacotherapy remains the most widely used method to control chronic cancer pain. The three categories of analgesic medications most commonly used are nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, and adjuvant agents, such as tricyclic antidepressants and corticosteroids.[1] The remainder of this article will focus on the use of opioids.
Opioids in Cancer Pain
Opioid analgesics are the most effective analgesics for severe pain and the mainstay of therapy for cancer patients with pain.[5] Morphine, hydromorphone, fentanyl, and oxycodone have been the opioids most commonly used in the United States.[1] Hydrocodone, methadone, tramadol, and tapentadol are some of the other medications used. The success of opioid therapy depends on the expertise of the prescriber who is knowledgeable about the nuances of the pharmacologic features among the various opioids before making an appropriate selection for each patient.[2]
The largest group of opioids is the morphine-like agonists. Morphine remains a prototype opiate analgesic against which all other drugs are compared. Despite its clinical utility, the associated side effects led to attempts to develop molecules with similar analgesic action without the management challenges.[1] Over the last century, numerous opiate drugs have been synthesized and the vast majority fall into the mu category (i.e., they target the mu opioid receptor [MOR]). Initially, all mu opioids were thought to act through a single class of opioid receptors, but subsequent research has identified genetic locations for several mu opioid receptor subtypes. To date, at least 25 variants of the mu receptor have been identified in mice, 8 in rats, and 11 in humans.[3] Although mu opioids share many pharmacological characteristics, there are differences.[6]
Opioid Rotation
The concept of multiple mu receptors may help explain the variability in individual response to various opioids, the differences in side effects among patients, incomplete cross-tolerance among various mu opioid analgesics, and the clinical utility of opioid rotation.[5-6] Opioid rotation is now a widely accepted approach to poorly responsive pain. If side effects with one opioid are significant, an improved balance between analgesia and side effects might be achieved by changing to an equivalent dose of an alternate opioid.[1] Rotation between two or three opioids is often required to obtain satisfactory long-term pain control.[5] One survey found that approximately 20% of patients rotate through three or more opioid medications before achieving an acceptable balance.[4] A systematic review of existing literature on opioid rotation performed in 2006 found that this approach results in clinical improvements in greater than 50% of patients with chronic pain who experience a poor response to one opioid.[6] Expanding the currently limited number of sustained-release oral opioids would provide clinicians with increased treatment options and dosing flexibility.
Long-Acting Opioids
Opioids are used to treat moderate to severe pain.[4] Long-acting opioids are indicated for the treatment of chronic pain and offer a number of advantages compared with shorter-acting agents. Sustained-release formulations provide more predictable serum levels, resulting in more predictable pain relief and the avoidance of mini-withdrawals caused by fluctuating opioid levels. In addition, they are easy to use and improve compliance rates. Overall, there is greater patient satisfaction and less reinforcement of drug-seeking behavior; patients do not need to be taught, for example, to take medications every four hours.
In a study of cancer patients treated with long-acting and short-acting opioids, patients in the long-acting opioid group had significantly lower pain intensity scores (P = 0.008) and better quality of life than patients in the short-acting opioid group, but other studies found long-acting and short-acting opioids conferred similar analgesic benefits. In a randomized, double-blind study of patients with cancer pain, patients taking sustained-release oral morphine reported significantly less tiredness than those taking IR oral morphine.[7]
There are reasons why prescribers might select long-acting analgesic agents over short-acting agents for their patients: long-acting formulations may provide analgesic benefit for 12–24 hours, long-acting agents may provide sufficiently long pain control to permit uninterrupted sleep, and long-acting agents reduce frequent dosing, which patients may prefer and which might improve compliance which, in turn, could improve analgesic relief. There appears to be widespread clinical acceptance of long-acting opioids, as evidenced by the fact that 23 million extended-release (ER) prescriptions were dispensed in the United States in 2009.[7]
Currently, there are a number of long-acting agents available. ER formulations are available for single-entity opioids, such as hydrocodone, hydromorphone, morphine, oxycodone, and tapentadol. Transdermal buprenorphine and transdermal fentanyl may also be considered long-acting single-entity opioid analgesics.[7]
Oral morphine sulfate controlled release (CR) (MS Contin®, Purdue Pharma, L.P., Stamford, CT) and oxycodone hydrochloride CR (OxyContin®, Purdue Pharma, L.P., Stamford, CT) have a duration of action of approximately 8 to 12 hours.[5] These agents are equally effective with a similar side effect profile. However, the addiction potential is different for morphine and oxycodone. Because of the street value, surveillance is warranted in patients who are prescribed oxycodone. Recent advances in abuse deterrent formulations may alleviate the drug abuse to some extent.
Long-Acting Morphine
Morphine is also available as a twice daily (Kadian®; Embeda,® King Pharmaceuticals, Bristol, TN) and a once-daily Avinza® (King Pharmaceuticals, Bristol, TN) capsule formulation that can be broken to release the morphine-containing pellets. These pellets can be sprinkled on applesauce for patients who are unable to swallow the capsule. Importantly, the pellets cannot be chewed, crushed, or dissolved because of the risk of a rapid release and absorption of a potentially fatal dose of morphine (Kadian® Prescribing Information; Avinza® Prescribing Information). In the case of Embeda, there is the risk of releasing an encapsulated dose of naltrexone (capsule strength at a morphine:naltrexone ratio of 25:1), potentially resulting in opioid withdrawal and cessation of analgesia. This is the first opioid formulation available with abuse deterrent technology.
Transdermal Fentanyl
Duragesic® fentanyl transdermal system (Janssen Pharmaceutica Products, L.P. Titusville, NJ) patch provides continuous opioid delivery for 72 hours. It is now also available in a generic version. When using the fentanyl transdermal system, it is important to provide short-acting pain medication for the first 8-12 hours before effective plasma concentrations are achieved. In 2007, the Food and Drug Administration (FDA) issued a public health advisory warning healthcare professionals about the safe use of the fentanyl transdermal system. Likewise, care must be exercised when patients are switched from fentanyl patches to another opioid analgesic, as it will take about 12 hours for the plasma concentration of fentanyl to decrease.
Oxymorphone
Oxymorphone hydrochloride (Opana®, Endo Pharmaceuticals Inc., Chadds Ford, PA) is one of the newer opioids available. It is a semisynthetic mu opioid agonist that produces a more rapid onset of action and greater analgesic potency compared with its parent compound, morphine.[10] Oxymorphone is available as 5 mg and 10 mg immediate release tablets and 5, 7.5, 10, 15, 20, 30, and 40 mg ER tablets. It is also supplied as a 1 mg/mL ampule for IV use. Studies in healthy men and women have shown that single and multiple dose administration results in a linear and dose-proportional increase in pharmacokinetics for oxymorphone. Peak concentrations are reached within 30 minutes.[11] A study in 300 patients with moderate-to-severe postsurgical pain demonstrated that oxymorphone reaches peak clinical effects approximately 45 minutes after administration.[12] This attribute of oxymorphone makes the immediate release formulation a good option for the management of breakthrough pain in cancer patients.
Abuse-deterrent extended release opioid formulations
- XARTEMISTM XR (previously known as MNK- 795, Mallinckrodt Brand Pharmaceuticals, Dublin, Ireland) is the first immediate-release (IR)/extended-release (ER), abuse-deterrent formulation comprised of a fixed-dose combination of oxycodone (7.5 mg) plus acetaminophen (325 mg) that is available on the market.[7,13]
- Embeda (morphine sulfate and naltrexone extended-release capsules)
- Hysingla ER (hydrocodone extended-release tablets, Purdue Pharma, Stamford, CT)
- Targiniq (oxycodone and naloxone extended-release tablets, Purdue Pharma)
- Xtampza ER (oxycodone extended-release capsules, Collegium Pharmaceuticals. FDA approved Sept. 2015)
Comparative Studies
In the cancer pain population, there were two studies that compared the efficacy of oxymorphone to oxycodone CR and morphine CR. Overall, the pain relief obtained was consistent among the opioids tested, and the observed adverse effects were typical of opioids. In one study by Gabrail and colleagues, following a 3-to-10 day titration period, 40 patients completed both a 7 to 10 day double-blind phase and the same length crossover period.[14] Oxymorphone ER and oxycodone CR had clinically similar average pain intensity ratings (2.5 ± 1.3/10 and 2.8 ± 1.3/10, respectively). Also, the amount of rescue medication used by each of the study groups was low and comparable: the oxymorphone patients took an average daily dosage of 16.6 mg morphine immediate release (range 0–90 mg) while the oxycodone group used 12.6 mg (range 0–75 mg). Once the patients had stabilized, equianalgesic dose ratios were calculated to be 2:1 oxymorphone ER to oxycodone CR.
In a second prospective, open-label, pilot study, cancer patients with moderate or severe pain who were stabilized on either morphine CR or oxycodone CR were safely and rapidly converted to oxymorphone ER at a lower milligram dose with no decrease in analgesic effectiveness or increase in adverse events. Long-term follow-up in a small group of cancer patients for about one year showed little tolerance to oxymorphone based on the mean average pain intensity analog scale (VAS) and a “good-to-excellent” pain relief rating by 80% of patients based on a categorical scale (Data on File. Endo Pharmaceuticals Inc., Chadds Ford, PA). Based on these results in small numbers of patients, additional trials of oxymorphone ER in chronic cancer pain are warranted.
This study of cancer patients also showed that although the incidences of nausea (18%) and vomiting (11%) were similar to that seen with other opioid analgesics, a lower incidence of constipation (11%) than is generally associated with opioid therapy could be achieved by integrating a bowel regimen into the study protocol. These results were duplicated in a 12-week study of low back pain, which also used a bowel regimen; 19.6% of the patients had nausea, whereas only 11.6% of them experienced constipation and only 8.8% experienced vomiting.
Pharmacokinetics and Pharmacodynamics of Opiates
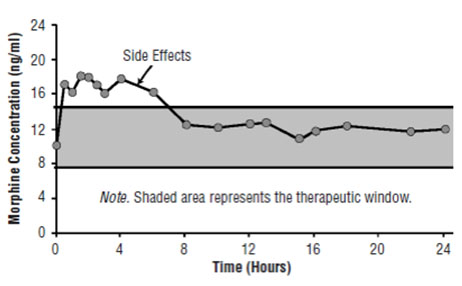
Figure 1A. Pharmacokinetic and Pharmacodynamic Relationships for Opiates: Illustration of Concepts. Plasma Concentrations Rise Above the Therapeutic Window Causing Side Effects.

Figure 1B. Pharmacokinetic and Pharmacodynamic Relationships for Opiates: Illustration of Concepts. Plasma Concentrations Fall Below the Therapeutic Window Resulting in Loss of Efficacy.

Figure 1C. Pharmacokinetic and Pharmacodynamic Relationships for Opiates: Illustration of Concepts. Therapeutic Window is Opened to Improve Patient Outcomes.
Understanding the pharmacokinetics and pharmacodynamics of opiates is imperative to providing patients with effective pain relief and avoiding adverse effects such as sedation, nausea, and vomiting.[10] Efforts are directed at staying within the therapeutic window to avoid side effects that occur when drug concentrations rise above those necessary for pain relief (Figure 1A) and loss of efficacy when plasma levels are too low (Figure 1B). The goal is to attempt to open the therapeutic window to improve outcomes for patients (Figure 1C). This can usually be achieved within two weeks using a small dose of a long-acting opioid and allowing the patient free access to short-acting opioids. Later, titrate the patient to a higher dose. This approach enables not only a widening of the therapeutic window but also avoidance of side effects.
Moreover, it has become critically important for the clinician prescribing opioids to also consider the metabolic pathways of the available opioids, the enzymatic systems used in the hepatic and intestinal metabolism[16] by medications synchronously prescribed to treat other conditions, and the genetic characteristics of a patient that may yield rapid, slow, or poor metabolizers of different drugs, including opioids.[17]
Breakthrough Pain

Figure 2. Steady-State Pharmacokinetics of Oxycodone CR Twice Daily: Illustration of Concepts. Breakthrough Pain Due to End-of-Dose Failure.
Breakthrough pain is defined as a transitory increase of more severe pain over relatively well-controlled baseline pain.[18-19] The reported incidence of breakthrough pain ranges between 16% and 95% in patients with persistent pain.[20] There are three types of breakthrough pain: end-of-dose failure, incident pain, and spontaneous/idiopathic pain.[20] End-of-dose failure occurs when plasma concentrations fall below the therapeutic window (Figure 2). To avoid end-of-dose failure, a short-acting opioid should be administered or the dosing interval should be decreased. It is important to talk to patients about patterns of breakthrough pain.
Incidental and idiopathic breakthrough pain both occur despite appropriate plasma levels in patients whose pain was previously well controlled. This type of true breakthrough pain, often severe, rises quickly and usually lasts between 30 and 45 minutes before subsiding. A medication with a rapid onset of action such as oral transmucosal fentanyl or a fentanyl buccal tablet is needed to control this type of pain has been suggested.[2] However, a physiopathological approach is again indicated to determine the source of breakthrough pain. Patients with paravertebral masses and foraminal nerve invasion frequently experience episodes of breakthrough pain. Since this pain is neuropathic in nature, the use of opioids, even ultra-short acting, are not likely to be effective in this setting. At this point, there is not a short-acting antineuropathic agent available. However, in the future, transnasal ketamine may be effective in this setting.
Overall, when dealing with breakthrough pain, it is important to determine whether the pain is end-of-dose failure or incidental and treat appropriately. Even if the patient is pain free but has a poor quality of life, the pain regimen needs to be adjusted. Prior to dose titration, pain scores and rescue medications should be monitored along with psychosocial variables, including sleep. The long-acting opioid should be adjusted based on the pain scores and the amount of rescue medication used.
Immediate-release fentanyl formulations for breakthrough pain
- Actiq (oral transmucosal fentanyl citrate, Teva Pharmaceuticals, Petah Tikva, Israel)
- Fentora (fentanyl buccal tablet, Teva pharmaceuticals)
- Abstral (fentanyl sublingual, Galena Biopharma/ProStrakan, Portland, OR)
- Subsys (sublingual fentanyl, Insys Therapeutics, Chandler, AZ)
- Onsolis (fentanyl buccal film, BioDelivery Sciences International, Inc., Raleigh, NC)
- Lazanda (fentanyl nasal spray, Depomed Inc., Newark, CA)
- Ionsys (fentanyl iontophoretic transdermal system, The Medicines Company, Parsippany, NJ)
See the National Cancer Institute’s PDQ® information on Opioids for selected opioid analgesics, routes of analgesic medication administration, and routes of fentanyl administration.
Dose Titration
An important question raised in the opioid literature is the conversion rate among the various drugs. Most conversion data presented in reference tables are derived from older studies that were not designed for determining relative potencies.[14] Yet, an understanding of equianalgesic doses is important when titrating long-acting opioids. One practical approach is the “rule of 2” (Table 2). Assuming a morphine dose of 100 mg/24 h and dividing it by two, one arrives at a dose equivalent of 50 mg/24 h for oxycodone/hydrocodone. Dividing it by four, one reaches the fentanyl (25 mcg/h) and oxymorphone (25 mg/24 h) dose equivalents. Dividing it by eight, one obtains the equianalgesic dose of 12 mg/24 h for hydromorphone. This method allows for relatively quick conversions. Although on the conservative side, these quick conversions should place the patient’s plasma levels in the therapeutic range. However, the patient should be contacted within a day or two to determine if further titration is required.
| Oxycodone-hydrocodone/oral | 50 mg/24 h |
| Fentanyl | 25 mcg/h |
| Oxymorphone | 25 mg/24 h |
| Hydromorphone/oral | 12 mg/24 h |
| Methadone/oral | 10-60 mg/24 h |
Although methadone is a very effective and inexpensive medication, it provides a unique challenge because there are no good dosing guidelines and no easy conversion rates.[14][21] For patients on a moderate opioid dose, we usually convert to 10 mg of methadone every six hours for the first week and then decrease the dose to 8 mg every six hours. If the patient is on higher opioid doses, the starting methadone dose is 20 mg every 8 hours for the first week.
Patients on methadone need to be monitored closely. One study found a correlation between the daily dose of methadone and the QTc interval in 17 patients who experienced torsade de pointes.[22] Of note, the relationship persisted after adjusting for the clinical variables known to be associated with QT-segment prolongation.[17] In all patients who receive methadone, it is imperative that baseline EKG testing be performed. In patients receiving high doses, EKGs should be performed monthly. In addition, electrolyte levels should be monitored, particularly in patients on medications that induce magnesium and potassium loss, including chemotherapeutic agents such as carboplatin and cisplatin. In November 2006, the FDA released an alert memorandum about this information. The memorandum resulted in the addition of a black box warning to the product labeling for methadone manufactured as Dolophine® Hydrochloride CII (Roxane Laboratories, Columbus, OH).
Important Considerations in the Use of Opioids
In general, long-term opioid use decreases cortisol levels, which may be why patients experience lassitude and lack of energy. Additionally, opioids decrease prolactin, luteinizing hormone, follicle stimulating hormone, testosterone, and estrogen levels, which in turn can cause osteoporosis.[20,23,24] This problem can be overcome in men by prescribing testosterone either IM or gel form. However, reduced sex hormones are more problematic in women, and a consult with an endocrinologist may be warranted to address the risk of osteoporosis.
Morphine should be administered cautiously and in reduced dosages in patients with severe renal or hepatic insufficiency, Addison’s disease, hypothyroidism, prostatic hypertrophy, or urethral stricture, and in elderly or debilitated patients. It is not unusual for cancer patients with renal dysfunction to experience myoclonus and hyperalgesia following morphine administration (MS Contin® Prescribing Information).
Another important consideration when administering opioids is the effect on cognitive function.[10] Side effects such as sedation, dizziness, and mental clouding interfere with activities that demand alertness, especially driving. Although driving is not advisable at the beginning of treatment, studies have shown that cognitive function (including the ability to drive and operate machinery) is often adequate in patients taking stable, moderate doses of opioids for chronic pain.[26-27]
Codeine, dihydrocodeine, and hydrocodone must be converted to morphine, the active metabolite, in order to exert an analgesic effect. The conversion to morphine is driven by enzyme CYP2D6. Without this enzyme, there is no conversion and little pain relief.[28] Some patients, particularly African-Americans, have reduced, or absent, CYP2D6 function.[29] In addition, other commonly used medications, including fluoxetine, haloperidol, and paroxetine, can inhibit CYP2D6 function resulting in a lack of pain relief.[29]
Managing the Poorly Responsive Patient
If the opioid administered is not providing benefit to the patient, it should be discontinued. Deciding when a patient is more harmed than helped by an opioid is one of the greatest challenges in pain management.[5] When evaluating the poorly responsive patient, there are a number of considerations. One of the first considerations is evidence of abuse or diversion. If there is no evidence of abuse or diversion then increasing the dose of the current opioid or switching to a different opioid should be considered. As previously discussed, opioid rotation based on “pharmacogenetics” is a strategy that clinicians are more frequently implementing. If there is a neuropathic component to the pain, then the patient may benefit from neuropathic pain medications such as tricyclic antidepressants or anticonvulsants.[5]
If these maneuvers are ineffective then other interventions should be investigated including intraspinal administration, alternative treatments such as biofeedback and other psychological therapies, herbal therapy, and acupuncture.
Summary
The management of cancer pain can be a challenging endeavor and many patients do not receive adequate palliation. An understanding of the various etiologies of cancer and the different types of pain they can produce is essential for appropriate therapeutic intervention.[1]
When strict regulatory controls were placed on opioids in the 1940s, there was a backlash against their use and cancer pain was greatly undertreated.[27] Yet opioids are the most effective analgesics and an important component of pain management in cancer patients. The individual variability in response to different opioids has important implications in clinical practice, and opioid rotation has been shown to be useful for opening the therapeutic window and establishing a more advantageous analgesia-to-toxicity ratio.[14,21] It has been suggested that a therapeutic repertoire of at least three opioid drugs should be available for the management of chronic pain in patients with cancer.[12]
Key Points
- Although up to 99% of cancer pain can be controlled, approximately 42% of patients do not receive adequate palliation.
- A physiologic approach to cancer pain management is required to determine if the pain is visceral, somatic, or neuropathic in nature.
- Approximately 10%–20% of patients rotate through three or more opioid medications before achieving an acceptable balance of efficacy and side effects.
- A therapeutic armamentarium of at least three different opioids should be available for the management of cancer pain.
References
- National Comprehensive Cancer Network (NCCN). (2007). Clinical Practice Guidelines in Oncology. Adult cancer pain. V.1.2007. Available at: www.nccn.org. Accessed: August 1, 2007.
- de Leon-Casasola, O.A., & Lema, M.J. Cancer pain. In: Wylie & Churchill-Davidson’s A Practice of Anesthesia. 7th Ed. Edited by Healy, T.E.J. & Knight, P.R. Arnold Publishers, London, England 2003:1255-1265.
- Oliver, J.W., Kravitz, R.L., Kaplan, S.H., & Meyers, F.J. Individualized patient education and coaching to improve pain control among cancer outpatients. J Cln Oncology 2001;19:2206-2212.
- de Leon-Casasola, O.A., & Lema, M.J. Cancer pain. In: Wylie & Churchill-Davidson’s A Practice of Anesthesia. 7th Ed. Edited by Healy, T.E.J. & Knight, P.R. Arnold Publishers, London, England 2003:1255-1265.
- Ballantyne, J.C. Chronic pain following treatment for cancer. The role of opioids. The Oncologist 2003;8:567-575.
- Pasternak, G.W. Insights into mu opioid pharmacology. The role of mu opioid receptor subtypes. Life Sciences. 2001;68: 2213-2219.
- Pergolizzi Jr., J.V., Taylor Jr.,R, Raffa R.B. The Potential Role of an Extended-Release, Abuse-Deterrent Oxycodone/Acetaminophen Fixed-Dose Combination Product for the Treatment of Acute Pain. Advances in therapy. 2015; 32 (6): 484-495.
- Kadian Prescribing Information.
- Avinza Prescribing Information.
- McNicol, E., Heroics–Mahler, N., Fisk, MR., et al. Management of opioid side effects in cancer-related and chronic no cancer pain: A systematic review. J Pain. 2003;34:231-256.
- Adams, M.P., & Ahdieh, H. Single- and multiple-dose pharmacokinetic and dose-proportionality study of oxymorphone immediate-release tablets. Drugs RD 2005;6:91-99.
- Cherny, N.J., Chang, V., Frager, G., et al. Opioid pharmacotherapy in the management of cancer pain: a survey of strategies used by pain physicians for the selection of analgesic drugs and routes of administration. Cancer. 1995;6:1283-1293.
- http://link.springer.com/article/10.1007/s12325-015-0213-5#page-1, accessed 12/21/15.
- Mercandante, S. & Bruera, E. Opioid switching: A systematic and critical review. Cancer Treatment Reviews. 2006;2:304-315.
- Endo Pharmaceuticals Inc.
- Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352:2211-2221
- Papaleontiou M, Henderson Jr. CR, Turner BJ, Moore AA, Olkhovskaya Y, Amanfo L, and Reid MC. Outcomes associated with opioid use in the treatment of chronic non-cancer pain. J Am Geriatr Soc.2010 Jul;58(7):1353-69.
- Gabrail, N.Y., Dvergsten, C., & Ahdieh, H. Establishing the dosage equivalency of oxymorphone extended release and oxycodone controlled release in patients with cancer pain: a randomized controlled study. Current Medical Research Opinion. 2004;20:911-918.
- Sloan, P., Slatkin, N., & Ahdieh, H. Effectiveness and safety of oral extended-release oxymorphone for the treatment of cancer pain: a pilot study. Support Care in Cancer. 2005;113:57-65.
- Payne, R. Recognition and diagnosis of breakthrough pain. Pain Med 2007;8:S3-S7.
- McNicol, E., Heroics–Mahler, N., Fisk, MR., et al. Management of opioid side effects in cancer-related and chronic no cancer pain: A systematic review. J Pain. 2003;34:231-256.
- Hale, M.E., Ahdieh, H., Ma, T., & Rauck. R. Efficacy and safety of OPANA ER (Oxymorphone Extended Release) for relief of moderate to severe chronic low back pain in opioid-experienced patients: A 12-week, randomized, double-blind, placebo-controlled study. J Pain 2007;8:175-184.
- Caraceni, A., Martini, C., & Zecca, E. Breakthrough pain characteristics and syndromes in patients with cancer pain. An international survey. Palliative Med, 2004;18:177-183.
- Krantz, M.J., Kutinsky, I.B., Robertson, A.D., & Mehler, P.S. Dose-related effects of methadone on QT prolongation in a series of patients with torsade de pointes. Pharmacotherapy 2003;23:802–805.
- MS Contin prescribing info
- Harris JD, de Leon-Casasola OA. Oral erythromycin and the risk of sudden death. (Letter). N Eng J Med 2005;352:301-304
- Ballantyne, J.C., & Mao, J. Opioid therapy for chronic pain. N Engl J Med 2003;349:1943-1953.
- Mendelson, J.H., Mendelson, I.E., & Patch, VS. Plasma testosterone levels in heroin addiction and during methadone maintenance. J Pharmacol Expl Ther 1975;192:211-217.
- Supernaw, R.B. CYP2D6 and the efficacy of codeine and codeine-like drugs. American Journal of Pain Management. 2001;11:30-31.
Leave a commentOrder by
Newest on top Oldest on top