Problem-Based Learning Discussion: Pain Management in Patients Undergoing Mastectomy and Axillary Surgery

Editor's note: We encourage submissions of deidentified cases for discussion in future issues. Send cases to asraeditor@asra.com. Would you like to share your opinions on cases? Send your name, practice setting, and contact information to asraeditor@asra.com.

A 68-year-old woman presents for bilateral mastectomy with sentinel lymph node biopsy and possible axillary dissection. She suffers from hypertension, diet-controlled diabetes, obstructive sleep apnea, and obesity (120 kg, body mass index is 37). Medications include lisinopril and metoprolol. The patient's blood pressure is well controlled, her hemoglobin A1C is 5.6%, and she has been medically optimized by her primary care provider.
What multimodal analgesic plan would you normally use for this patient?
Warren: At my institution, the typical multimodal analgesic plan for this patient would include acetaminophen, opioids, and local anesthetic infiltration performed by the surgeon. Our surgeons typically instill local anesthetic through the drain and clamp the drain for an hour, after which the clamp is released. Patients are quite comfortable and do well. Our practice is to only perform paravertebral blocks for patients having additional immediate reconstruction, but on occasion for select patients we will also perform paravertebral or pectoralis nerve and serratus plane (PECS) blocks for simple mastectomy/axillary dissection. We also consider the use of perioperative gabapentin and celecoxib.
Pawa: At my institution, we have not implemented the preoperative administration of multimodal analgesics (acetaminophen, nonsteroidal anti-inflammatories, and gabapentinoids) despite having a large breast cancer caseload. I would therefore not administer anything preoperatively. My primary concerns here would be that her diabetes is controlled and that she omits her lisinopril on the day of surgery.
Feinstein: In the preoperative holding area, this patient would be offered a standardized oral multimodal regimen based on age and pertinent lab findings. The medications include gabapentin 800 mg (800 mg for those younger than 69 years and 400 mg for those older than 69 years); acetaminophen 1,000 mg; and celecoxib 400 mg (if GFR greater then 60 mL/min/1.73 m2, 400 mg for those younger than 69 years and 200 mg for those older than 69 years). The patient would also be offered a single-injection interfascial plane block prior to surgery.

What is your anesthetic plan for this patient? Do you need any additional information to formulate your plan?
Warren: I would like to have more information about the patient's functional status and at least a recent echocardiography test to review. I would confirm that her blood pressure is well controlled and that she has no unstable cardiovascular symptoms and no evidence or history of congestive heart failure. If her functional status is limited, I would want to review a recent stress test or transthoracic echo. In addition, I would ask whether she uses continuous positive airway pressure (CPAP) machine regularly, and if she had any previous anesthetic-related issues or history/symptoms of significant gastrointestinal reflux disease. I would include a thorough exam of the airway and auscultation of heart and lungs in my physical assessment.
Pawa: I would use my standard practice for mastectomy and axillary surgery and perform general anesthesia maintained by total intravenous anesthesia with a target-controlled infusion of propofol. For this patient, I would intubate the trachea to secure her airway. Analgesia would be delivered by siting bilateral single-shot, single-level paravertebral blocks, possibly with the addition of bilateral PECS blocks. I would be keen to minimize administration of any opiates. Prior to siting the paravertebral blocks, I would like to know whether the patient was on any antiplatelet or anticoagulant drugs or whether she had any other potential contraindications to siting them. It would be useful to know the severity of the obstructive sleep apnea and her reliance on CPAP so that I could plan her postoperative destination. In all likelihood, I would request a high-dependency bed for her postoperative recovery. Finally, it would be useful to know how well her diabetes and blood pressure were controlled and whether she had any evidence of end-organ damage.
Feinstein: If no other information was revealed during a preoperative assessment, the patient would receive an interfascial plane block and general anesthesia with an endotracheal tube for this surgery. If the patient was not a candidate for regional anesthesia, a lidocaine infusion would be used throughout the case.
What pharmacologic agents would you choose for maintenance of general or monitored anesthesia?
Warren: My general anesthetic plan would be either total intravenous anesthesia with propofol or a volatile anesthetic with sevoflurane and possibly nitrous oxide. I might also consider using low-dose ketamine to potentially reduce opioid use. I do not use remifentanil infusions and rather administer a long-acting opioid during the case. Neuromuscular blockade would most likely be obtained with succinylcholine followed by rocuronium. A monitored anesthesia care (MAC) protocol might include low-dose propofol, midazolam, and fentanyl. Dexmedetomidine is another option during MAC, although not one that I personally use.
Pawa: My general anesthesia would be maintained by a target-controlled infusion of propofol, guided by a depth of anesthesia monitor such as the bispectral index. The choice of drug is largely based on the work from two studies.[1,2]
If the patient was amenable and motivated, I would consider using the propofol infusion as MAC. Our group has published our experience with this perioperative strategy.[3]
Feinstein: Unless she has significant comorbidities that warrant avoidance of general anesthesia, the patient would be induced with lidocaine, fentanyl, propofol, and succinylcholine. Our surgeons request that no long-acting muscle relaxants be used in the setting of axillary dissection. Dexamethasone would be given shortly after induction as prophylaxis for nausea. Anesthesia would be maintained with sevoflurane in an air-oxygen mixture. Near emergence, the patient would also receive ondansetron.
What regional anesthesia technique would accompany your planned anesthetic?
Warren: Given the patient's size, an ultrasound-guided paravertebral block may be challenging, although I would attempt it depending on the anatomy visualization. If paravertebral block anatomy is not favorable, then I would perform ultrasound-guided PECS blocks (I, II, serratus plane).
I would use bilateral, ultrasound-guided paravertebral blocks with a in plane transverse intercostal approach (linear probe 6–13 mHz), depositing bupivacaine 0.5% with 1:400,000 epinephrine, 20 mL each side (total 40 mL volume). I typically perform only a single-level block between T2–T4. If the plan is to conduct surgery under a sole regional anesthetic technique, I would probably perform two level injections bilaterally at T2–T3, T5–T6 with bupivacaine 0.375% with epinephrine 1:400,000, with 10–12 mL at each site (total of 40–48 mL). If visualization is difficult, I would attempt a more oblique ultrasound probe position or consider a sagittal paramedian approach. Recognizing that analgesia may be incomplete for the axillary dissection, I might also consider the addition of a PECS II block (ie, ultrasound-guided PECS I, II, serratus plane block using either bupivacaine 0.25% or 0.375% with 60–70 mL volume).
Pawa: My practice is largely based on anesthesia for breast cancer. The block I site the most for mastectomy surgery is the thoracic paravertebral, and I routinely site bilateral blocks for bilateral surgery. I occasionally supplement with a PECS I or II block, depending on the surgery and the patient. It is my standard practice to site paravertebrals preoperatively under a small amount of sedation, usually a bolus dose of midazolam (1–2 mg), possibly with a small amount of intravenous fentanyl (25–75 mcg). All of my paravertebrals are ultrasound guided, and with patients of this size, I position patients in the semiprone position and use a transverse, in-plane approach to the paravertebral space with an 18g Tuohy needle. If performing analgesic blocks, I usually use weight-appropriate doses of 0.25–0.5% levobupivacaine.
Feinstein: PECS blocks are offered preoperatively as our primary postoperative analgesic. The injection used for this block is 25 mL of bupivacaine 0.25%, with 2 mg of dexamethasone per side. One-third of the injectate is given between the pectoralis major and minor and two-thirds between the pectoralis minor and serratus anterior.
In our institution, patients receiving these blocks are routinely able to avoid intravenous opioids and often require minimal oral opioids in the first 24 hours. PECS and serratus blocks may offer improved analgesia, compared to other blocks for mastectomy, because of the unique blockade, which includes both lateral cutaneous branches of the intercostal nerves and some terminal branches of the brachial plexus. Some concern has been raised about the potential blockade of the long thoracic nerve when using PECS and serratus blocks, but our breast surgeons feel comfortable proceeding with the block. Additionally, PECS blocks are arguably easier to perform, faster, and safer than paravertebral blocks.

Would you consider insertion of a thoracic epidural as an alternative to paravertebral or pecs blocks?
Warren: Certainly, a mid- to high-thoracic epidural would be a good alternative to a paravertebral or PECS block; however the incidence of hypotension, motor weakness, and urinary retention is much greater than with a more peripheral approach. Pneumothorax is usually the risk that pushes many to prefer thoracic epidural over paravertebral block, but the incidence of pneumothorax, or clinically significant pneumothorax, is exceedingly low with a paravertebral block at my institution. Furthermore, recent publications have supported the low incidence of pneumothorax with ultrasound-guided paravertebral block.
Pawa: I would not consider siting a thoracic epidural in these patients for a number of reasons. First, I am an ultrasound convert, and it is difficult to site thoracic epidurals in real time with ultrasound guidance. I also do not regularly site thoracic epidurals for any other parts of my practice, so it is a landmark technique with which I am becoming less proficient. Second, I believe that the risk profile associated with a landmark-guided thoracic epidural is worse than that of an ultrasound-guided paravertebral, especially in the obese population. Third and perhaps most significantly, if I consented my regular patients for an epidural for breast surgery, because of the stigma associated with epidurals, most patients would refuse to have one instead of either a paravertebral or a PECS block.
Feinstein: Thoracic epidurals are not used for mastectomy at our institution. Multiple concerns, including hypotension, urinary retention, pruritus, contraindications with blood thinners, and potential difficulties with ambulation, along with an inpatient requirement, have eliminated the technique from our practice.
Do you routinely use local anesthetic additives as part of your regional anesthetic?
Warren: I do not add adjuncts to my local anesthetic. Epinephrine is added as a vascular marker but probably does contribute to prolonging the duration of block effect as well.
Pawa: As a general rule, I do not use additives perineurally for paravertebral blocks. The only exception is when performing anesthesia for awake breast surgery. In those circumstances, I use lidocaine with epinephrine in addition to levobupivacaine to speed onset and reduce absorption. For most cases I administer intravenous dexamethasone, which I think has a beneficial effect despite the lack of evidence for paravertebral blocks.
Feinstein: Dexamethasone is a standard additive in our single-injection blocks. Meta-analysis of dexamethasone as an additive has shown an approximately 50–100% increase in duration of analgesia. Despite being off-label, dexamethasone has a long history of safe use in neuraxial as well as peripheral nerve blocks.
How do the patient's obesity and obstructive sleep apnea impact your intraoperative management plan?
Warren: Obstructive sleep apnea and obesity would probably spur me to use a regional anesthetic if this patient were undergoing a procedure at my institution that typically does not include regional anesthesia. It is important to recognize that obesity is likely to make a regional technique more challenging and may put patients at increased risk for pneumothorax. A good multimodal plan with local anesthetic infiltration and a balanced anesthetic can also work well. Pawa: Obesity is not a problem restricted to North America and is certainly something that we are dealing with more frequently in the United Kingdom. The combination with obstructive sleep apnea focuses the mind and strengthens my resolve to use as opiate sparing a technique as possible. I mentioned previously that I prefer using a transverse, in-plane technique for paravertebral block insertion in obese patients. I would aim to avoid the perioperative administration of an intravenous opiate for either block insertion or intraoperative rescue. Instead, I would use bolus doses of ketamine for intraoperative rescue. I would also consider extubating the patient immediately onto CPAP to minimize problems in the postoperative period. If she were suitable for a general anesthesia-free technique (to have the surgery under blocks and sedation), I would consider the intraoperative use of her own CPAP or high-flow nasal oxygen.
Feinstein: Intraoperative management of patients with sleep apnea and obesity tends to focus on airway management, ventilation and oxygenation difficulties, and extubation. Using multimodal analgesics and regional anesthesia to minimize the use of opioids and other sedatives is valuable in decreasing the risk of respiratory compromise.
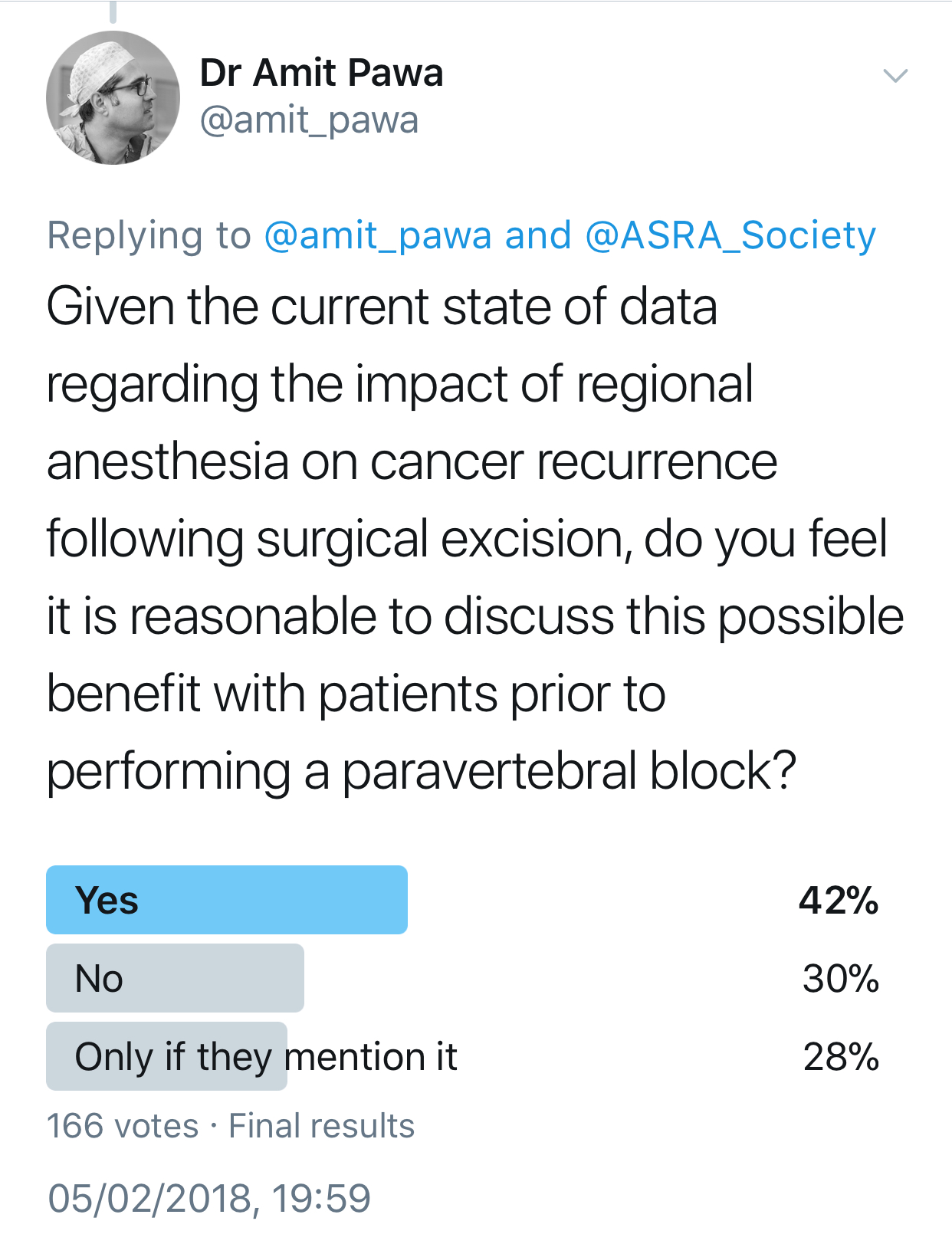
Your patient mentions having read that general anesthesia makes cancer recurrence more likely. How would you respond?
Warren: The jury is still out, and it appears that the avoidance or limiting the use of opioids may be the important factor in cancer recurrence.
Pawa: I would be honest and explain that presently, no definitive evidence backs up such a statement. Interest had been raised in breast cancer, based on some work performed 12 years ago.4 The working hypothesis is that avoiding general anesthetic drugs and opiates and providing anesthesia via regional techniques using local anesthesia may retain the immune system's integrity and have an impact on reducing cancer recurrence. This is currently being investigated thoroughly via a randomized, multicenter trial, but at present no conclusive evidence exists either way.
Feinstein: Although animal models have shown some risk of recurrence with certain anesthetics, previously published retrospective human studies have not demonstrated conclusive results when evaluating the risk of recurrence and exposure to volatile anesthetic. Likewise, the theory that regional anesthesia may reduce the risk of recurrence has also not been proven, but ongoing studies will hopefully resolve these questions.

Following a thorough discussion with the patient, you agree to provide general anesthesia and perform bilateral paravertebral blocks at T3 and T5 with 10 mL 0.25% bupivacaine at each level to provide postoperative analgesia. After the last injection, spread of local anesthetic and needle tip location are noted to be subpleural. The needle is immediately removed, and the patient neither coughs nor complains of dyspnea.
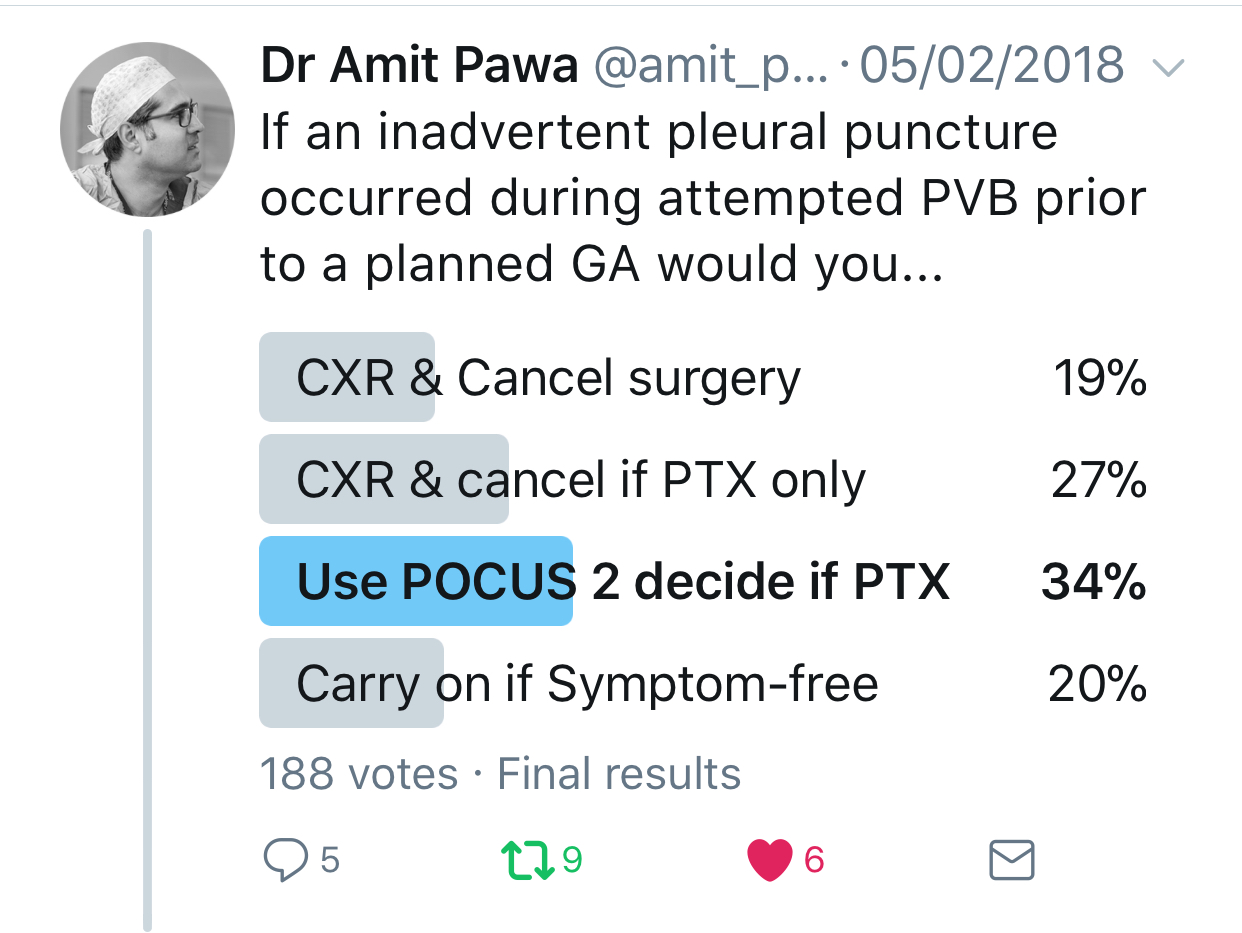
In the setting of an inadvertent pleural puncture, how would you elect to proceed?
Warren: I would proceed with the procedure under sole regional technique.
Pawa: Most subpleural needle placements do not lead to pneumothorax. Also, intrapleural administration of local anesthetic is a recognized mode of analgesia (although not one I usually practice). I think that in the absence of cough or dyspnea, the sensible approach is to perform point-of-care ultrasonography to assess for pneumothorax (the absence of lung sliding or the observation of a lung point). If any doubt exists, a chest x-ray can also be performed.
Feinstein: After a known pleural puncture, the patient's lungs would be examined with ultrasonography and chest x-ray to look for a pneumothorax. Although ultrasonography has been shown in some studies to be more sensitive in diagnosing a pneumothorax, a chest x-ray can determine the extent of pneumothorax and help dictate care from the percentage of pneumothorax.
Additionally, pleural puncture does not always lead to pneumothorax. If the needle is not open to room air and there is no insult to the parenchyma of the lung, then a pneumothorax is unlikely to occur.
Assuming chest x-ray and/or ultrasonography fail(s) to demonstrate any pneumothorax, would you proceed with the planned general anesthetic?
Warren: This becomes a bigger discussion with the surgeon and the patient. If there is no evidence of pneumothorax (sonographic or radiographic assessment), then I would proceed with the planned general anesthetic, keeping in mind that positive pressure ventilation may create a pneumothorax during the surgery. Possible options include placing a prophylactic pigtail chest tube preoperatively, proceeding with surgery with a plan to place the pigtail tube (or chest tube) intraoperatively if it becomes necessary, or rescheduling the case.
Pawa: Yes, I would elect to proceed with the planned anesthesia and surgery.
Feinstein: If the patient remains asymptomatic, we would proceed to the operating room with increased vigilance for the possible development of intraoperative pneumothorax. To lessen the risk, we would avoid nitrous oxide and maintain spontaneous ventilation.
You elect to proceed with the case and it is conducted uneventfully. Four hours following the block, the surgical team asks if to inject a local anesthetic into the wound and, if so, how much. How do you respond?
Warren: I would ask the surgical team members what they are trying to accomplish. Paravertebral block duration usually exceeds the local infiltration duration. However, if they feel strongly about wound infiltration, I would ask that they avoid injecting into a blood vessel and use bupivacaine 0.25% with epinephrine (to reduce vascular absorption) up to 40–50 mL. I would request a smaller-volume injection if the patient has a reduced ejection fraction or hepatic or renal insufficiency.
Pawa: I am not entirely sure why the further injection of local anesthetic into the wound would be necessary after insertion of bilateral paravertebral blocks, unless there was axillary wound extension and I had not performed PECS blocks. As a result, although it is theoretically possible, I would ask them to refrain from injecting any further local anesthetic.
Feinstein: The surgical team may use additional local anesthetic. The dose depends on the amount used in the preoperative block, time since the preoperative block, and the patient's risk factors for toxicity (eg, heart and liver failure, age, weight). We would direct the surgical team to address the areas at risk for insufficient coverage from the paravertebral blocks (eg, axilla). We use a maximum of 3 mg/kg in a 4-hour time frame before considering additional local usage.
The patient is extubated uneventfully and taken to the postanesthesia care unit. You are called to evaluate her for 8/10 bilateral axillary pain. How would you evaluate and manage?
Warren: A physical exam is necessary to make sure that no physical reason is causing pain (eg, bleeding, hematoma development, arm ischemia, nerve compression) and to determine the actual site of pain.
Sensory innervation of the axilla is supplied by the lower brachial plexus (C8–T1–medial cutaneous nerve of the arm) and may not be covered with a paravertebral block, although it is not uncommon for cervical spread of local anesthetic with a paravertebral block as evidenced by frequent development of Horner syndrome. The medial arm/axilla is also supplied by the intercostobrachial (T2) nerve, which should be covered with a paravertebral block. A PECS II block can usually cover the intercostobrachial nerve successfully, but a brachial plexus block that covers the medial cord is necessary to block the medial cutaneous nerve of the arm. The superficial cervical plexus (supraclavicular nerves) may also contribute to sensory innervation of the axilla and anterior chest. I would consider performing additional blocks to cover what might have been missed.
Pawa: I would take a pain history and examine the patient to assess the cause of pain. I would also ask for a surgical consult. The possible differential diagnoses for pain would be surgical site pain because of inadequate scar analgesia in axilla (PECS block was not performed), pain from transection or electrocautery to intercostobrachial nerve, acute hematoma formation, and nonsurgical site pain from excessive shoulder abduction. If a PECS block had not been performed, I would clamp the drains for 30 minutes and perform bilateral PECS II blocks. If a PECS block had been performed, I would attempt to administer small intravenous boluses of ketamine or to instill local anesthesia via the drains.
Feinstein: After evaluating the patient at bedside and confirming the bilateral axillary pain, we would order PRN opioids as well as a single-dose intravenous ketorolac.
What could you have done to prevent the patient's significant axillary pain?
Warren: I could have performed a low-dose brachial plexus block (low interscalene, supraclavicular, or infraclavicular approach) to cover the medial cutaneous nerve of arm, although I would be wary of causing phrenic nerve paresis in an obese patient with obstructive sleep apnea (interscalene and infraclavicular blocks). An infraclavicular block is also challenging in morbidly obese patients. Alternatively, I could have done bilateral PECS II/intercostobrachial nerve block to cover the axilla or possibly bilateral superficial cervical plexus blocks.
Pawa: Depending on the cause, a number of options are available. Insertion of preemptive PECS blocks would be my first choice. The other potential strategies are preoperative administration of a gabapentin, not cutting the intercostobrachial nerve, or infiltrating the axillary wound with local anesthesia as the surgeons suggested.
Feinstein: In our experience, the PECS and serratus blocks enable better axillary analgesia. These interfascial plane blocks provide brachial plexus blockade that is missed when a paravertebral or epidural is used.
References
- Abdallah FW, Morgan PJ, Cil T, et al. Ultrasound-guided multi-level paravertebral blocks and total intravenous anesthesia improve the quality of recovery after ambulatory breast tumor resection. Anesthesiology. 2014;120:703–713.
- Wu J, Buggy D, Fleischmann E, et al. Thoracic paravertebral regional anesthesia improves analgesia after breast cancer surgery: a randomized controlled multicentre clinical trial. Can J Anesth. 2015;62:241–251.
- Pawa A, Wight J, Onwochei DN, et al. Combined thoracic paravertebral and pectoral nerve blocks for breast surgery under sedation: a prospective observational case series. Anaesthesia. 2018;73(4):438–443.
- Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis?Anesthesiology. 2006;105:660–664.
- Woodworth GE, Ivie RM, Nelson SM, et al. Perioperative Breast Analgesia. A qualitative Review of Anatomy and Regional Techniques. Reg Anesth Pain Med. 2017;42:609–631.
Leave a commentOrder by
Newest on top Oldest on top