Clinical Implications of IV Lidocaine Infusion in Preoperative/ Acute Pain Settings
Introduction
Lidocaine (xylocaine) was first introduced by Torsten Gordh in the 1940s and, since then, the clinical application of lidocaine has expanded beyond that of local anesthesia, making use of its systemic analgesic, anti-inflammatory, and anti-hyperalgesic effects.1-2 Systemic lidocaine infusion has been used as an analgesic adjunct for the management of acute perioperative pain in many clinical settings. An ever-growing body of evidence supporting enhanced recovery after surgery (ERAS) protocols has led to a trend toward opioid-sparing multimodal analgesia, which may include intravenous (IV) lidocaine.
Mechanism of Action
Postoperative pain is due to a combination of inflammatory and neuropathic processes. The systemic inflammatory response to surgical trauma leads to neuroinflammation, decreasing the firing threshold of A-δ and C-fibers and acute postoperative pain. This increases the release of glutamate, which in turn increases N-methyl-D-aspartate (NMDA) receptor activation, leading to hyperalgesia and allodynia. In the chronic setting, this leads to activation of microglia and astrocytes in the dorsal horn of the spinal cord; in turn, this enhances the persistent release of proinflammatory cytokines and algesic mediators.3-4
The observed clinical benefits of lidocaine exceed its half-life by greater than 5.5 times (8.5–24 hours) after discontinuation of the infusion.2 This is long after lidocaine has been metabolized to its nonbiologically active byproducts, pointing to alternative mechanisms beyond its local anesthetic properties. In vitro studies have demonstrated the modulatory effect of lidocaine on potassium channels, calcium channels, G-coupled protein receptors, NMDA receptors, and the glycinergic system. Systemic administration of lidocaine tends to decrease IL-1δ, TNF-α, ICAM-1, mucosal COX-2, and plasma prostaglandin E2. Such mechanisms are thought to contribute to the anti-neuroinflammatory effects of lidocaine and may explain its clinical benefits in the management of acute and chronic pain.3
Polymorphonuclear granulocytes (PMNs) have a pivotal role in the release of proinflammatory cytokines and reactive oxygen species (ROS) ramping up the migration of neutrophils through a feed-forward loop. Lidocaine inhibits the priming of PMNs by lysophosphatidic acid (LPA) and platelet-activating factor (PAF).3,5 Schmidt et al described the inhibitory effect of lidocaine on PMN adhesion and migration.6 This may contribute to the modulatory effect of lidocaine on the “sterile inflammation” seen in trauma and surgery.2,3,5,6
Some animal studies demonstrate suppression of polysynaptic C-fibers and wide dynamic range (WDR) neurons, which also may play a role in the anti-neuropathic pain properties of lidocaine.3
CLINICAL APPLICATION
According to the 2016 review in the British Journal of Anaesthesia, perioperative lidocaine infusion correlated with decreased visual analogue scale (VAS) pain scores at 1 to 4 hours and 24 hours postoperatively.2 Other benefits mentioned by several systematic reviews include decreased opioid requirements, reduced nausea, decreased time to first flatus, and decreased length of hospital stay.2,7,8 The greatest benefit of perioperative lidocaine infusion was seen in patients undergoing laparoscopic and open abdominal surgery.2 Similar benefits have been observed genitourinary, breast surgery, thoracic surgery, and spine surgery.4,8–10 We do not recommend perioperative lidocaine infusion for cardiac surgery, obstetrics, or hip surgery. This conclusion is based on the inability of the corresponding studies to demonstrate significantly different outcome measures when compared to nonlidocaine groups.11–13

There are few studies published regarding postoperative lidocaine infusion. At our institution, we retrospectively compared postoperative lidocaine infusion to epidural analgesia with bupivacaine and hydromorphone up to postoperative day (POD) 6 in 216 patients (108 in each group) undergoing major abdominal surgery. Lidocaine was associated with fewer episodes of postoperative hypotension, nausea, vomiting, pruritus, and urinary retention and earlier removal of the urinary catheter after surgery.14 Interestingly, the lidocaine group had higher IV and PO opioid consumption (determined by equianalgesic doses of IV morphine). We did not observe a significant difference in pain scores after POD 2. Another retrospective study in our institution compared 52 patients receiving IV lidocaine as part of the colorectal ERAS protocol (Figure 1) to 52 patients who received standard intraoperative and postoperative lidocaine infusion (see below for dosing) undergoing colorectal surgery (B. Naik, personal communication, October 19, 2016). The ERAS group had lower opioid consumption on POD 1; however, the analysis of POD 1 pain scores was inconclusive. On POD 2 and beyond, lidocaine alone was non-inferior to lidocaine given as part of the ERAS protocol in terms of postoperative pain. The ERAS group demonstrated decreased time to ambulation and discharge, as well as urinary catheter removal.
Safety/Side Effects
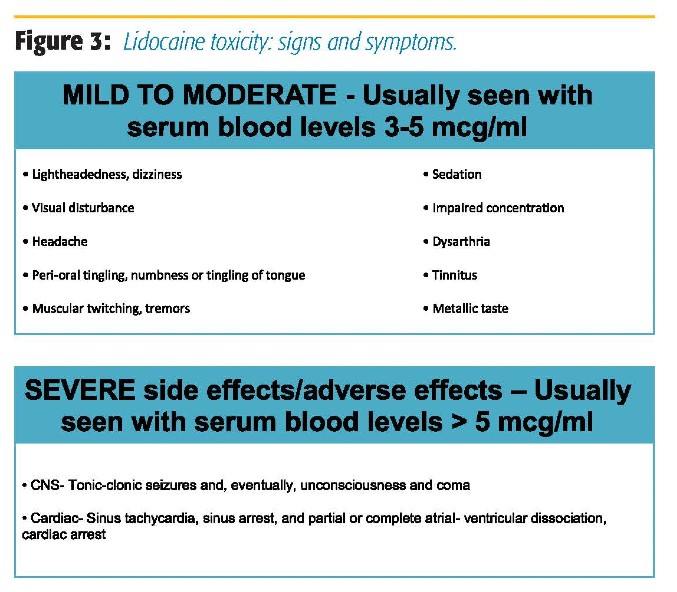
The safety profile of IV lidocaine has been previously described.2–4,7,15 Mild side effects, including dizziness and visual disturbances, have been described in some meta-analyses.5,10 Serious side effects, such as neurologic changes and cardiac toxicity, are exceedingly rare.2,5,15 Anecdotally, some practitioners report delayed emergence among patients receiving lidocaine infusion. However, to date, no association has been demonstrated between perioperative lidocaine infusion and delayed postoperative care unit (PACU) discharges.16 Blunting of airway reactivity to the endotracheal tube might be an explanation for the perceived delayed emergence.
Dosing
Evidence regarding the optimal dose for IV lidocaine is lacking. Doses that produce serum lidocaine concentrations equivalent to epidural lidocaine infusion (approximately 1 micromolar) have been associated with decreased pain, nausea, opioid requirement, and shorter duration of ileus and length of stay after abdominal surgery.5,17 Bolus doses of 0.5 to 1.5 mg/kg followed by 1.5 to 3 mg/kg/hr infusion resulted in decreased postoperative VAS pain scores and cumulative opioid consumption in several clinical trials. Infusion rates of 2 mg/kg/hr or higher are associated with lower pain scores and opioid consumption when compared to lower doses.2,3,8 In our institution, an infusion rate of 40 mcg/kg/min after 1–1.5 mg/kg bolus is used perioperatively as part of our ERAS protocols. The infusion rate is decreased to 5–10 mcg/kg/min at the end of the surgery and continues at the same rate until POD 2. Our acute pain management lidocaine infusion protocol uses a 0.5 mg/min starting dose with a maximum of 1 mg/min for adults, and doses between 15 to 25 mcg/kg/min for pediatric patients <40m kg.
Approval and Development of the Acute Pain Management Lidocaine Infusion Protocol at the University of Virginia
It is well known that factors such as postoperative pain, ileus, nausea, and vomiting contribute to prolonged hospital stay and increased cost.2,8 These realities have allowed our anesthesiology and acute pain attendings to present IV lidocaine as a relatively safe intervention aimed to improve such outcomes. IV lidocaine is now routinely used for analgesia in acute pain management and ERAS protocols at our institution. This protocol was established through the interdisciplinary efforts of members of the anesthesia, surgery, and nursing staff. Since the approval of the protocol, several quality improvement projects and publications by our department have strengthened the advocacy for the use of lidocaine in the perioperative/acute pain settings.
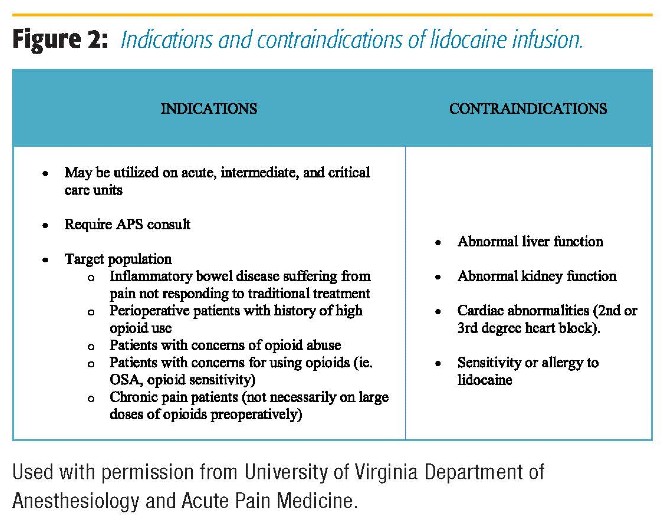
In our institution, intraoperative lidocaine infusion is routinely used in open and laparoscopic abdominal surgery, urology, GYN, spine, orthopedic, and thoracic surgery in both ERAS and nonERAS patients. The decision regarding continuing lidocaine infusion postoperatively for non-ERAS patients is made after discussions with the surgical team and the Acute Pain Service (APS). APS is routinely consulted for the start of and management of lidocaine infusions for postoperative and nonsurgical patients (trauma, chronic pain, etc.). Lidocaine infusion can be and is often used in conjunction with lumbar and thoracic epidurals in both ERAS and non-ERAS patients, as long as the epidural infusion does not contain local anesthetic. Figure 2 presents the indications and contraindications for IV lidocaine. APS rounds daily on these patients, and patients are monitored for signs of lidocaine toxicity (Figure 3). Recommendations regarding dosing of the lidocaine infusion, as well as the multimodal pain regimen, are made during rounds. There are specific instructions for nurses to monitor for signs of toxicity while caring for patients receiving lidocaine infusions. Other than mentioned, we do not require additional physiologic monitoring other than unit protocol (ie, patients do not require a monitored bed to be on lidocaine infusion). A member of the APS team is available for nursing staff to contact with any questions or concerns. We have had no adverse events related to lidocaine infusion at the doses recommended in our protocol.
Our APS team takes an active role in nursing engagement (see “Acknowledgement”) by including nurses in the daily rounds on patients receiving IV lidocaine. This allows communication between APS and the nursing staff, which has been important to the successful launch of the University of Virginia’s acute pain lidocaine infusion protocol as well as its implementation in our ERAS protocols.
Final Word
There are several limitations to the studies supporting the use of perioperative lidocaine infusion. These include a lack of large double-blinded placebo-controlled trials, as well as limited data regarding the optimal dosing and duration of treatment. According to ClinicalTrails.gov, at this time, there are 48 open clinical trials regarding various analgesic applications of IV lidocaine infusion. The growing interest in the ERAS concept has inspired a wave of various investigational endeavors in pharmacology and pathophysiology of acute and perioperative pain. This has enhanced the role of the anesthesiologists as expert consultants and forerunners of research in this field. The future holds the key to the Pandora’s box of new interventions aimed at improving the perioperative experience of patients.
Acknowledgement
Approval and initiation of the lidocaine infusion protocol within our institution are the results of the valiant efforts of our APS nurse coordinator, Steve Morton, RN.
References
1. Gordh T, Gordh T, Lindqvist K. Lidocaine: the original local anesthetic. Anesthesiology. 2010;113:1433–1437.
2. Weibel S, Jokinen J, Pace N, et al. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br J Anaesth. 2016;116(6):770–783.
3. van der Wal SE, van den Heuvel SA, Radema SA, et al. The in vitro mechanisms and in vivo efficacy of intravenous lidocaine on the neuroinflammatory response in acute and chronic pain. Eur J Pain. 2016;20(5):655–674.
4. Hollmann MW, Herroeder S, Kurz KS, et al. Time-dependent inhibition of G protein-coupled receptor signaling by local anesthetics. Anesthesiology. 2004;100(4):852–860.
5. Kirillova I, Teliban A, Gorodetskaya N, et al. Effect of local and intravenous lidocaine on ongoing activity in injured afferent nerve fibers. Pain. 2011;152(7):1562–1571.
6. Schmidt R, Yamashita M, Ando K, Tanaka Y, Cooper J, Patterson G. Lidocaine reduces reperfusion injury and neutrophil migration in canine lung allografts. Ann Thorac Surg. 1996;61(3):949–955.
7. Sun Y, Li T, Wang N, Yun Y, Gan TJ. Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2012;55(11):1183–1194.
8. Farag E, Ghobrial M, Sessler DI, et al. Effect of perioperative intravenous lidocaine administration on pain, opioid consumption, and quality of life after complex spine surgery. Anesthesiology. 2013;119(4):932–940.
9. Terkawi AS, Sharma S, Durieux ME, Thammishetti S, Brenin D, Tiouririne M. Perioperative lidocaine infusion reduces the incidence of post-mastectomy chronic pain: a double-blind, placebo-controlled randomized trial. Pain Physician. 2015;18:E139–46.
10. Lauwick S, Kim DJ, Mistraletti G, Carli F. Functional walking capacity as an outcome measure of laparoscopic prostatectomy: the effect of lidocaine infusion. Br J Anaesth. 2009;103(2): 213–219.
11. El-Tahan MR, Warda OM, Diab DG, Ramzy EA, Matter MK. A randomized study of the effects of perioperative i.v. lidocaine on hemodynamic and hormonal responses for cesarean section. J Anesth. 2009;23(2):215–221.
12. Martin F, Cherif K, Gentili ME, et al. Lack of impact of intravenous lidocaine on analgesia, functional recovery, and nociceptive pain threshold after total hip arthroplasty. Anesth. 2008;109(1):118–123.
13. Mathew JP, Mackensen GB, Phillips-Bute B, et al.; Neurologic Outcome Research Group of the Duke Heart Centre. Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke 2009;40(3):880–887.
14. Terkawi AS, Tsang S, Kazemi A, et al. A clinical comparison of intravenous and epidural local anesthetic for major abdominal surgery. Reg Anesth Pain Med. 2016;41(1):28–36.
15. Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology. 2000;93:858–875.
16. McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs. 2010;70(9):1149–1163.
17. Inoue R, Suganuma T, Echizen H, Ishizaki T, Kushida K, Tomono Y. Plasma concentrations of lidocaine and its principal metabolites during intermittent epidural anesthesia. Anesth. 1985;63(3):304–310.