The Role of Minimally Invasive Lumbar Decompression (MILD) In The Management of Symptomatic Lumbar Spinal Stenosis: A Problem-Based Learning Discussion
Mr. CM, a 65-year-old male, presents to the clinic with a six-month history of progressively worsening lower back pain. He rates his pain as an eight out of ten on the pain severity scale, and states that the pain is largely dull and achy in nature but he does endorse an intermittent sharp, shooting pain to his bilateral legs. He reports tingling, numbness, and heaviness in both lower extremities which gets progressively worse with prolonged walking, often requiring him to take frequent breaks when ambulating. He often feels unsteady on his feet and he now uses a cane for assistance. Leaning forward on his cane and sitting down provide some relief. He notes that the pain is worsened by prolonged time standing straight or lying down at times. He mentions one episode of urinary urgency where he was unable to reach the bathroom in time but has not experienced further occurrences. He is referred to your pain management clinic for further evaluation.
1. Develop an appropriate differential diagnosis for this patient presenting with symptomatic lumbar spinal stenosis and describe the process of differentiating common pain generators in the lumbar spine.
In this patient presenting with both axial and radicular low back pain, a comprehensive history and physical exam is integral to correlating the patient’s symptoms to their pathology. In fact, numerous studies have demonstrated that there is poor correlation between degenerative changes in the lumbar spine on imaging studies and intensity of pain symptoms.1 Therefore, data from imaging studies should be viewed as a component of our diagnostic toolkit, particularly useful in the planning of procedural interventions and to confirm our suspected pathology.
In our patient with progressive paresthesias and lower extremity heaviness with prolonged ambulation, neurogenic and vascular claudication should be on the differential. “Red flag signs” when evaluating a patient with low back pain include the presence of bowel/bladder incontinence or retention, bilateral lower extremity weakness not related to prolonged standing activity, presence of saddle anesthesia or sudden onset gait dysfunction. Any of these symptoms warrant clinical suspicion for cauda equina and conus medullaris syndromes. Emergent imaging and surgical evaluation for emergent decompression are warranted.1 A strong clinical suspicion for and rule out of rare extra spinal causes of similar presentations including thoracic aortic or renal artery dissections is important, particularly in patients presenting with acute onset symptoms.
A purposeful interview to delineate exacerbating and relieving factors of the patient’s lumbar back pain will reveal a positive “shopping cart sign,” or an improvement in patient’s neurogenic claudication symptoms with lumbar flexion. In addition to range of motion testing of the lumbar spine, strength, deep tendon reflex, and sensation testing of the lower extremities is important to assess for neurological dysfunction. The use of special tests such as the straight leg raise and seated slump test are both useful for confirming the presence of nerve root compression from a herniated intervertebral disc. Facet joint loading testing as well as provocative tests of the sacroiliac joint and hip may help to differentiate between concomitant pathologies in the region which may present with similar symptoms.
2. Describe the pathophysiology responsible for neurogenic claudication and compare/contrast with vascular claudication.
Symptomatic lumbar spine stenosis is often described as neurogenic or pseudoclaudication and is characterized by axial low back pain, buttock, thigh or calf pain with lower extremity cramping, weakness and sensory changes worsened with ambulation.2 Symptoms are often exacerbated by activities that involve spinal extension such as prolonged upright walking, standing, and sitting. 2 Walking up stairs, bending forward, and forward flexion with ambulation often improve lumbar spinal stenosis symptoms. 2 This symptomatology is pathognomonic for lumbar spinal stenosis and has been termed “shopping cart sign” as the patient endorses leaning or bending over a shopping cart or other support structure while standing or ambulating to reduce symptoms.3 In spinal flexion, the diameter of the spinal canal increases and the ligamentum flavum is flattened, helping to relieve the compression of neural elements. 3
The pathophysiology of lumbar spinal stenosis and neurogenic claudication is multifaceted. Most neurogenic claudication symptoms are thought to originate from nerve tissue compression and relative nerve tissue micro-ischemia.5 The ligamentum flavum is a thick ligament connecting the adjacent vertebrae from C2 to S1, consisting of elastic (80%) and collagen (20%) fibers.6 Ligamentum flavum hypertrophy, is an important contributor to lumbar spinal stenosis. This process is associated with VEGF mediated angiogenesis following mechanical stress leading to loss of elastin and increased fibrosis and hypertrophy of the ligament by fibroblasts.6 Neural element irritation may take place in several ways, including direct mechanical compression, increase in cerebrospinal fluid pressure, or by adjacent tissue inflammation.7 Kobayashi et al describe mechanical forces of spinal stenosis contributing to subarachnoid space occlusion which results in venous congestion and increase endoneurial pressure.
Vascular claudication is a symptomatic presentation associated with peripheral artery disease and stems from pain in the lower extremities secondary to demand ischemia during exertion such as walking.4 A careful evaluation of the patient’s symptom presentation as well as risk factors for vascular disease or dysfunction are crucial to distinguish between vascular and neurogenic pain.
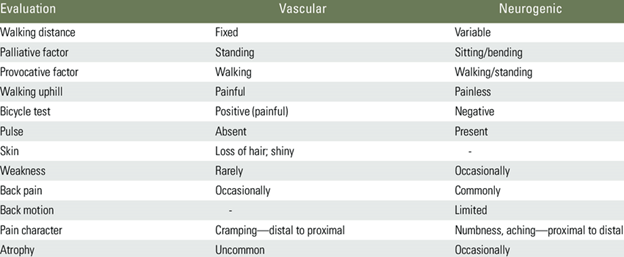
Table outlining differentiating features of vascular and neurogenic claudication.4
3. What imaging studies would you order and what findings would you expect to see in this patient with symptomatic lumbar spinal stenosis?
You order standing A/P and dynamic (flexion/extension) lateral X rays, which revealed multilevel spondylosis, loss of intervertebral disk height, and flattening/loss of lumbar lordosis. There was a grade 1 spondylolisthesis noted at L4/L5 which was stable in flexion and extension. Due to the severity of his symptoms, an MRI of the lumbar spine without contrast was also performed, showing extensive narrowing of the spinal canal throughout the lumbar region, worst at the L4/L5 intervertebral disc level with severe central, lateral and foraminal stenosis. At this level the hypertrophied ligamentum flavum measures 3.5 mm thick at its widest point, and the anterior-posterior diameter of the canal is noted to be 9 mm. There are no noted cord signal changes.
Lumbar spine X-ray is a common initial diagnostic test for routine low back pain. X-ray provides a cost-effective evaluation of the bony spine anatomy and generally includes an anterior-posterior (AP) and lateral view. Additional X-ray views such as oblique and flexion/extension views are often added for better evaluation of the pars interarticularis and dynamic stability of the vertebral column respectively. Patients with a higher degree of chronicity and complexity may require more advanced imaging such as magnetic resonance imaging (MRI) or computed tomography (CT).8 Lumbar MRI is the diagnostic study of choice for the evaluation of spinal stenosis and provides the finest resolution of soft tissue as well as the neural elements.8
In evaluation of lumbar spine stenosis, MRI is crucial in determining the vertebral level, severity of stenosis, stenotic comorbidities, and degree of ligamentum flavum hypertrophy.9 In the case of patient specific MRI contra-indications lumbar CT may be used, although with lower resolution of the neural elements and soft tissue structure of the spine. Patients with greater than 2.5mm of ligamentum flavum hypertrophy and mild-moderate lumbar spinal stenosis may be candidates for the MILD procedure.6 Patient selection and careful review of spine anatomy on advanced imaging are paramount to the success of the MILD procedure. With respect to diagnostic imaging, the ideal candidate for the MILD procedure may be a patient with MRI findings of mild-moderate lumbar spine stenosis due to ligamentum flavum hypertrophy (>2.5mm), with or without concomitant facet or disc disease and without severe canal or foraminal stenosis, spinal instability, or other previous spine surgery.
4. Given patient’s diagnosis of lumbar spinal stenosis with neurogenic claudication you would like to proceed with the MILD procedure. What criteria must be met for a patient to be a candidate for this procedure? What are some contraindications to undergo this procedure?
Treatment options for lumbar spinal stenosis range from conservative approaches to surgical interventions. Minimally invasive lumbar decompression (MILD) is a minimally invasive procedure suited for patients who have not responded to conservative therapies. The MILD procedure involves the removal of excess hypertrophic ligamentum flavum that contribute to the narrowing of the spinal canal.
Ideal candidates for MILD are patients with lumbar spinal stenosis and neurogenic claudication persisting for more than three months, who have failed conservative treatments. Additionally, MRI findings should indicate ligamentum flavum hypertrophy greater than 2.5 mm and central canal narrowing to less than 100 mm² cross sectional area.6
Not all patients are suitable candidates for the MILD procedure. Exclusionary criteria for the MILD procedure includes grade II or higher spondylolisthesis, significant epidural lipomatosis and severe neurogenic claudication with inability to ambulate greater than 10 feet.6 For this reason, any patient with risk or suspicion of spondylolisthesis should have a full evaluation with dynamic lumbar flexion/extension X-ray to ensure spinal stability prior to the MILD procedure.6 Additionally, patients with difficulty lying prone for the duration of the procedure, bleeding disorders or conditions affecting wound healing, such as diabetes or cancer should be evaluated carefully for suitability.6 There is ongoing debate in the literature regarding other factors such as spondylolisthesis, lateral recess stenosis, and the degree of ligamentum flavum hypertrophy, which may require individualized consideration.
For eligible patients, MILD offers several advantages. Importantly, spinal stability is preserved, as only a small portion of tissue is removed. According to Yuan and Yi10, no cases of post-procedure lumbar instability have been reported in clinical trials. Furthermore, the inner layer of the ligamentum flavum remains intact, preventing interference with the epidural space, thus reducing the risk of epidural hematomas or post-operative adhesions, which can occur in other spinal procedures. Additionally, the absence of intra-spinal adhesions allows future surgeries to proceed without complications arising from the MILD procedure. The risk of post-surgery neuropathic pain is low. Clinical trials have demonstrated that for ideal candidates, pain intensity can be reduced by approximately 50%. The overall effectiveness rate of the MILD procedure varies between 57.1% and 88%, according to multiple clinical studies.10
Although the MILD procedure is highly effective and minimally invasive, some patients may not achieve sufficient pain relief. This can occasionally be attributed to inadequate decompression or underlying neuropathic issues, though the incidence of inadequate decompression is low. As with any spinal procedure, theoretical complications include dural tears, intraoperative hemorrhage, epidural hematomas, nerve root injuries, and surgical infections. However, due to the minimally invasive techniques employed, these risks are rare and generally mild. Reported complications are typically limited to soreness at the surgical site and minor postoperative bleeding.10
5. Does this patient require an evaluation by a spine surgeon prior to performing this procedure? What characteristics/features would warrant a surgical referral?
For patients who do not respond to conservative treatment options, evaluation for potential surgical interventions may be warranted. The primary objectives of surgical intervention are to achieve optimal pain control, enhance functional capacity, and prevent further neurological deficits.11 Surgical options are typically indicated in cases of low back pain associated with neurological deficits or bowel and bladder dysfunction.12
The specific surgical procedure recommended depends on several factors, including the location of the stenosis and the stability of the spine assessed during surgery.12 Additional considerations include the degree of degenerative spondylolisthesis, the patient’s surgical history, and the presence of scoliosis or kyphosis.12 The choice of procedure should be made in consultation with a medical provider. For example, the MILD procedure may be particularly suitable for patients with ligamentum flavum hypertrophy predominantly affecting the posterior aspect of the ligament. Conversely, a decompressive laminectomy is an ideal choice for patients with central canal stenosis, as it involves removing the lamina to alleviate pressure on the spinal canal.
6. The patient has questions regarding how this procedure will be performed and the expectations following the procedure.
Minimally invasive lumbar decompression is a minimally invasive surgical procedure designed to debulk/remove excess hypertrophied ligamentum flavum (LF). The theory behind this procedure is to remove excess ligament that is connected between the vertebrae of the spine in order to relieve pressure or tension acting on the spinal cord.
Initially, MRI w/o contrast is performed before the procedure takes place to evaluate epidural space, level of thickness of LF, and identify major areas of lumbar stenosis producing clinical symptoms of neurogenic claudication as endorsed by the patient. On the day of operation, the patient is brought into an OR and usually put under light sedation with local anesthetic as well. The patient will be placed in prone position with a bolster to reduce lumbar lordosis at spinal levels. With the aid of fluoroscopic-guided imaging, the contralateral oblique and AP view is obtained to ensure vertebral bodies are squared off to ensure optimal laminar visualization. Next, an epidurogram can be performed to identify the border of epidural space in relation to LF.13 A small incision is made over selected inferior lumbar region; this will be 1 to 1.5 vertebral segments caudad to the target area.14 A trocar/cannula is an advanced cephalad carefully with firm pressure toward the dorsal surface of the spinal lamina and lateral to spinous process. Contralateral oblique view is obtained to confirm location.14 A MILD portal stabilizer is placed to lock the device into position. Subsequently, the trocar is removed, and a depth guide is placed to limit forward motion of the main apparatus. A bone rongeur is then used with imaging guidance on selected laminar bone in order to cut away bites of inferior/superior lamina which usually requires 3 passes each respectively.14 The goal is to obtain interlaminar access. The final portion of this procedure involves a tissue sculpter that is advanced toward the portal tip. For optimal results, approximately 3 scooping passes 3 times in performance when removing the LF. Each pass involves directing the tip of the sculptor into the interlaminar space toward the ventral aspect of superior lamina before squeezing on trigger. The procedure can also be performed on the contralateral side or another vertebral level.
Patients can typically resume normal activity post-procedure once sedation has worn off. Usually, a follow up visit is scheduled within a few weeks to evaluate the amount of symptom relief and for any potential neurological changes. Physical therapy can also be prescribed to optimally improve muscle strengthening and conditioning. Furthermore, improvement is generally noted within a few weeks and has been shown to be durable at 5y post-MILD procedure.15 Currently, there are relatively few evidence-based systematic reviews that discuss Warfarin being discontinued 5 days before surgery. For Xa inhibitors like Apixaban or Rivaroxaban, it is recommended to stop medications 3 days before surgery.16 For antiplatelet agents, it is recommended to stop 1 to 3 days before surgery. For Plavix, 5 to 7 days before surgery.16
7. What are potential post-procedural complications and standard post-procedure followup protocols? What is the safety profile of MILD?
The MILD procedure is generally well tolerated with a robust safety profile comparable to that of an epidural steroid injection.6 However, there are still some complications to be aware of that include but are not limited to neurological injury at performed level of procedure, dural tear, infection, or epidural hematoma.6 There is less concern for cauda equina syndrome as there have been no reported cases of this. Occasionally, patients may present with acute numbness or weakness that self-resolves. Given the nature of a MILD procedure involving removing ligamentum flavum which can be adhered to the dura mater and in close proximity to posterior epidural space, there is some risk with developing dural tears. In addition, an epidural hematoma can rarely develop in high risk elderly patients with a history of GI bleed or aspirin use. Furthermore, with any interventional procedure there is always an increased risk of infection; although infection rates are low in MILD procedures since there is no actual implantation, is it important to keep in mind post-op.
In a study by Mekhail et al, 75 patients with lumbar spinal stenosis underwent a MILD procedure. There were 0% reported intraoperative complications. Furthermore, there were 17.3% minor post-op complications that involved post-op pain, ecchymosis, and allergic dermatitis with 0% major complications.15
References:
1. Babińska A, Wawrzynek W, Czech E, Skupiński J, Szczygieł J, Łabuz-Roszak B. No association between MRI changes in the lumbar spine and intensity of pain, quality of life, depressive and anxiety symptoms in patients with low back pain. Neurologia i Neurochirurgia Polska. Published online December 11, 2018. doi:https://doi.org/10.5603/pjnns.a2018.0006
2. Bussières A, Cancelliere C, Ammendolia C, et al. Non-Surgical Interventions for Lumbar Spinal Stenosis Leading To Neurogenic Claudication: A Clinical Practice Guideline. The Journal of Pain. 2021;22(9):1015-1039. doi:https://doi.org/10.1016/j.jpain.2021.03.147
3. Hayes A, Herning M, Gonzalez-Snyder C. Musculoskeletal system. In: Occupational Therapy with Aging Adults. ; 2016:97-124. doi:https://doi.org/10.1016/B978-0-323-06776-8.00017-7
4. Lee SY, Kim TH, Oh JK, Lee SJ, Park MS. Lumbar Stenosis: A Recent Update by Review of Literature. Asian Spine Journal. 2015;9(5):818. doi:https://doi.org/10.4184/asj.2015.9.5.818
5. J.N. Blau, Valentine Logue, INTERMITTENT CLAUDICATION OF THE CAUDA EQUINA: An Unusual Syndrome Resulting from Central Protrusion of a Lumbar Intervertebral Disc, The Lancet, Volume 277, Issue 7186, 1961
6. Jain S, Deer T, Sayed D, et al. Minimally invasive lumbar decompression: a review of indications, techniques, efficacy and safety. Pain Manag. 2020;10(5):331-348. doi:10.2217/pmt-2020-0037
7. Kobayashi, S. (2014). Pathophysiology, diagnosis and treatment of intermittent claudication in patients with Lumbar Canal stenosis. World Journal of Orthopedics, 5(2), 134. https://doi.org/10.5312/wjo.v5.i2.134
8. Lateef, H., & Patel, D. (2009). What is the role of imaging in acute low back pain? Current Reviews in Musculoskeletal Medicine, 2(2), 69–73. https://doi.org/10.1007/s12178-008-9037-0
9. Hartman, J., Granville, M., & Jacobson, R. E. (2019). Radiologic evaluation of Lumbar Spinal Stenosis: The integration of sagittal and axial views in decision making for minimally invasive surgical procedures. Cureus. https://doi.org/10.7759/cureus.4268
10. Yuan, H., & Yi, X. (2023). Lumbar Spinal Stenosis and minimally invasive lumbar decompression: A narrative review. Journal of Pain Research, Volume 16, 3707–3724. https://doi.org/10.2147/jpr.s428112
11. Hennemann S, de Abreu MR. Estenose Degenerativa do Canal Lombar. Revista Brasileira de Ortopedia. 2020;56(01):009-017. doi:10.1055/s-0040-1712490
12. Sengupta, D. K., & Herkowitz, H. N. (2003). Lumbar spinal stenosis. Orthopedic Clinics of North America, 34(2), 281–295. https://doi.org/10.1016/s0030-5898(02)00069-x
13. Deer TR, Grider JS, Pope JE, et al. Best practices for minimally invasive lumbar spinal stenosis treatment 2.0 (MIST): Consensus guidance from the American Society of Pain and Neuroscience (ASPN). Journal of Pain Research. 2022;Volume 15:1325-1354. doi:10.2147/jpr.s355285
14. Deer TR. New image-guided ultra-minimally invasive lumbar decompression method: The mild® procedure. Pain Physician. 2010;1;13(1;1):35-41. doi:10.36076/ppj.2010/13/35
15. Mekhail N, Costandi S, Nageeb G, Ekladios C, Saied O. The durability of minimally invasive lumbar decompression procedure in patients with symptomatic lumbar spinal stenosis: Long‐term follow‐up. Pain Practice. 2021;21(8):826-835. doi:10.1111/papr.13020
16. Porto GBF, Jeffrey Wessell DO, Alvarado A, Arnold PM, Buchholz AL. Anticoagulation and Spine Surgery. Global Spine J. 2020;10(1 Suppl):53S-64S. doi:10.1177/2192568219852051