Lung Ultrasonography for the Regional Anesthesiologist
Note: This article originally appeared in the ASRA News, Volume 15, Issue 4, pp. 23-26 (November, 2015).
Section Editor: Melanie Donnelly, MD
Author
Stephen Haskins, MD
Assistant Attending Anesthesiologist
Hospital for Special Surgery
Clinical Instructor of Anesthesiology
Weill Cornell Medical College
New York, New York
One of the highly touted benefits of ultrasound-guided regional anesthesia (UGRA) is improved safety. Despite proficiency and advances in UGRA, many anesthesiologists still rely heavily on traditional imaging modalities such as x-ray to evaluate the lung for pathology. Ultrasonography of the lung to evaluate for pneumothorax has been shown to be more sensitive and specific than chest x-ray (sensitivity 88% vs 52%; specificity 100% vs 99%).[1] The Bedside Lung Ultrasound in Emergency (BLUE) Protocol by Lichtenstein and Mezière[2] has demonstrated that lung ultrasonography is an excellent tool to diagnose other lung pathology as well, such as asthma, chronic obstructive pulmonary disease, pneumonia, and pulmonary edema as seen in congestive heart failure (CHF) or acute respiratory distress syndrome (ARDS). In this article, we discuss basic lung ultrasonography for the regional anesthesiologist. By describing a clinical scenario, we will showcase a patient who could benefit from a bedside lung ultrasonography examination to rule out a pneumothorax as well as assess for pulmonary edema.
Clinical Scenario
An 82-year-old man is scheduled for an urgent open reduction and internal fixation of right proximal humeral fracture. He has a history of decompensated CHF and has been stabilized with dobutamine and furosemide infusions. He recently had a subclavian central venous catheter placed on the contralateral side for medication administration. While performing a supraclavicular block in the holding area with a junior resident, the patient develops acute respiratory distress. You are concerned about a new pneumothorax or potential worsening of his CHF. In this scenario, the use of ultrasonography to assess the lung can quickly help you distinguish between a pneumothorax and worsening CHF exacerbation.
Basics of Lung Ultrasonography
The first step to evaluate the lung is to confidently identify the pleura. This can be done with any ultrasound probe (Figure 1a); however, the small curvilinear probe is often used because the small footprint fits neatly in between two rib spaces, facilitating identification of the “batwing sign” (Figure 1b). For novices, the batwing sign is essential to locating the pleura. The batwing sign is two ribs (noted by their dark rib shadowing) and the bright line in between the ribs. That bright, shimmery, shiny, hyperechoic line is the pleura. Probe placement is incredibly important depending on the pathology being assessed. When evaluating for a pneumothorax, place the probe where air will most likely rise within the thorax. In a supine patient, a pneumothorax will be seen in between the second and third intercostal space midclavicular line (Figure 1c). If the patient is sitting up, scan at the apex of the lung; however, diagnosis may be more challenging. Note that this location is also ideal to assess for pulmonary edema, as the lung should be most aerated in this area. M-mode is a helpful modality for lung ultrasonography, particularly when assessing for pneumothorax. M-mode is a one-dimensional mode that plots out one slice of the ultrasound image as it changes over time. Almost every ultrasound machine has M-mode. Figure 1d shows the location of the M-mode buttons for two standard point-of-care ultrasound machines.
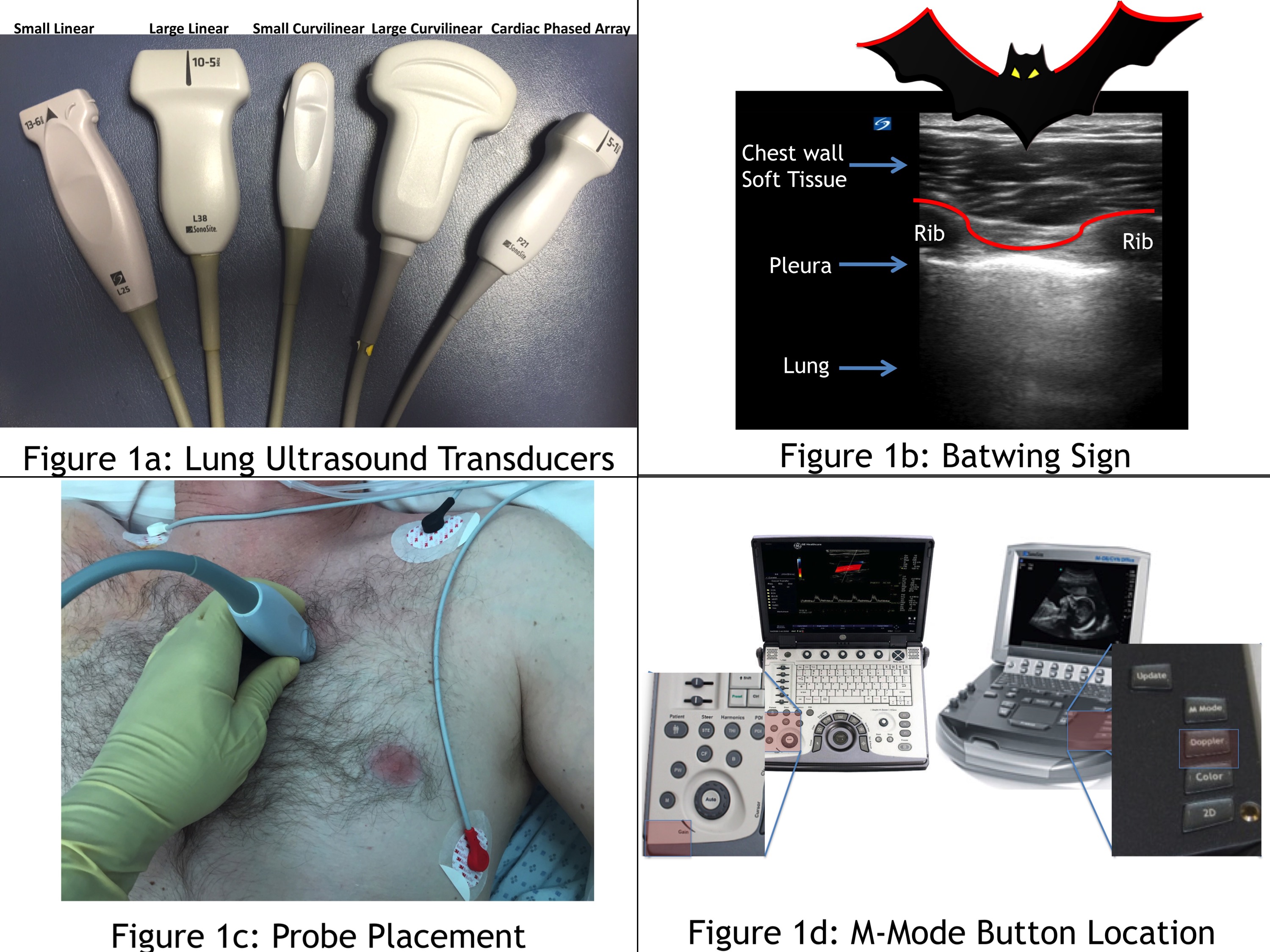
Figure 1: Lung ultrasound transducers (a). Batwing sign (b). Probe placement (c). M-mode button location (d)
What Am I Looking For?
The following signs can be used to rule out a pneumothorax with ultrasonography: lung sliding, lung pulse, B-lines, and the seashore sign. Lung sliding of the bright hyperechoic pleura is a back and forth or “to and fro” movement that corresponds with the patient’s breathing (either spontaneously or with ventilation).
Lung sliding demonstrates the lung’s dynamic visceral pleura sliding along the chest wall’s static parietal pleura. With a pneumothorax, air separates the two pleural layers and attenuation of the ultrasound waves, as they pass through the air layer, prevents visualization of the visceral pleura. Therefore, a pneumothorax causes the lung sliding to cease and the image of the pleura becomes static. Absence of lung sliding should significantly increase your suspicion for a pneumothorax.
Image Optimization Tips: Decrease depth and gain to improve visualization.
Lung pulse is similar to lung sliding; however, it is seen in patients who are apneic or taking shallow breaths. With apnea, the “to and fro” lung sliding is less clear, but the heartbeat can still move the two pleural layers. This movement is pulsatile and corresponds with the heartbeat. It is more prominently visualized in the left hemithorax. In an apneic patient, visualizing lung pulse can help you rule out a pneumothorax; however, absence of lung pulse alone does not increase suspicion for pneumothorax.
Image Optimization Tips: Decrease depth and gain to improve visualization. Place probe on left side of chest to better demonstrate with apnea.
B-lines are ultrasound artifacts that appear as comet-tails or rockets emerging from the pleural line and go all the way to the edge of the screen (Figure 2a). Visualization of a single B-line rules out a pneumothorax because it can only be seen with normal interaction between the visceral and parietal pleura. The B-line artifact is the result of an interaction between aerated lung and interstitial fluid. It is normal to see up to three B-lines in dependent areas of the lung (eg, the base of the lung) because the lungs typically have a small amount of interstitial fluid. The presence of more than three B-lines indicates an abnormal amount of interstitial luid as seen in CHF and ARDS (Figure 2b). A fully aerated lung will appear black, while a patient with significant interstitial fluid will have “white-out” with a significant number of B-lines in the lung.
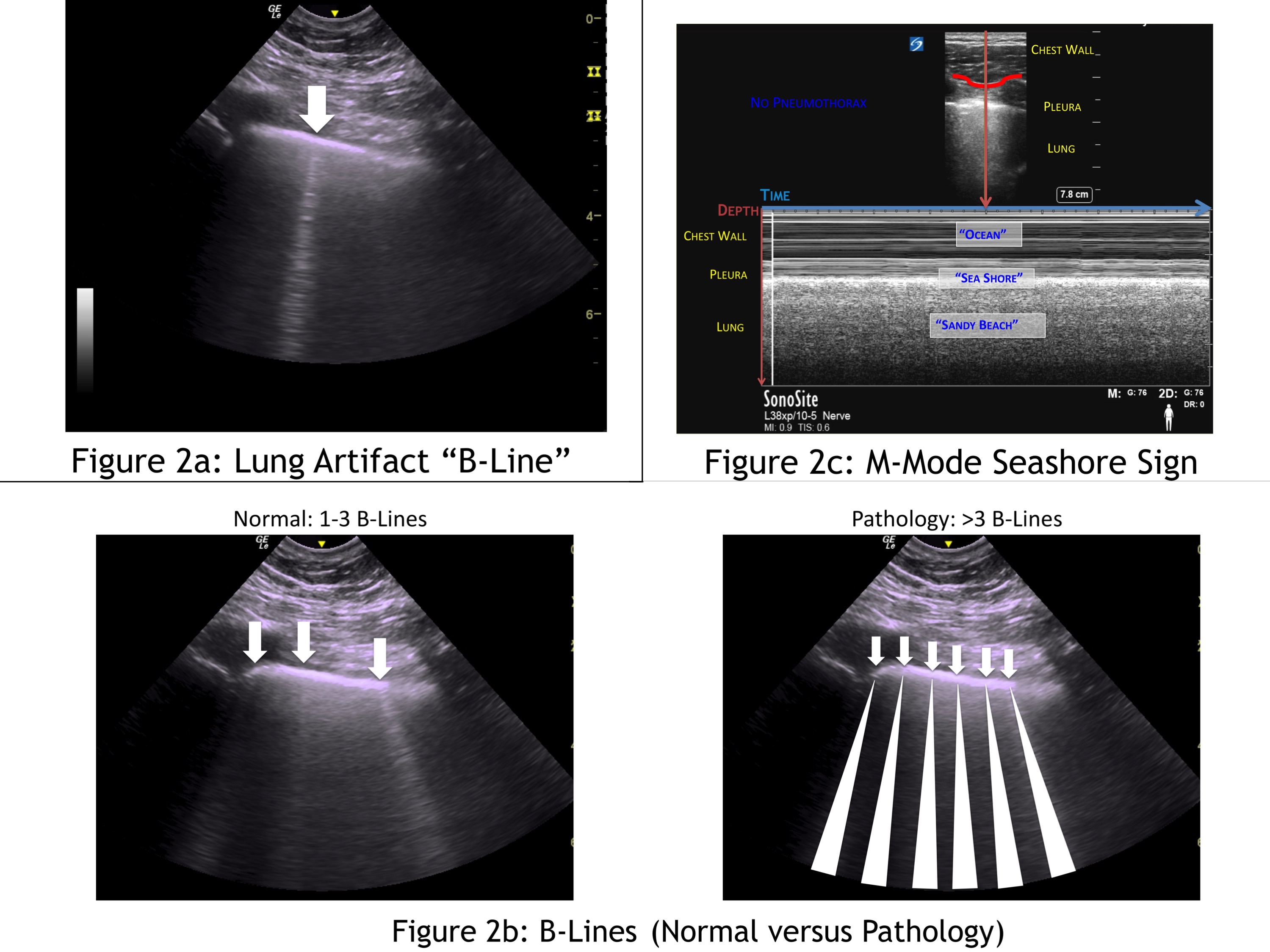
Figure 2: Lung artifact “B-line” (a). B-lines (normal versus pathology) (b). M-mode seashore sign (c)
Image Optimization Tips: Scan at the base (dependent area) of the lungs to visualize more B-lines.
The seashore sign is a normal finding when assessing the lung with M-mode. First, identify the batwing sign (Figure 1a) and then, using M-mode, assess between the two ribs. In M-mode, a onedimensional slice of the 2D image is plotted in the y-axis with the x-axis representing how it changes over time. In the near field (upper part of the image), minimal movement of the chest wall tissue appears as straight lines, which look like ocean waves. The bright pleural line is the divider between the chest wall, and the lung looks like a shore line. The lung, deep to the pleura, appears granular and sandy like a beach. A normal lung in M-mode therefore looks like ocean waves crashing onto a sandy beach; hence, the seashore sign (Figure 2c).
Image Optimization Tips: Increase depth and gain to better differentiate between chest wall and lung tissue.
Scanning Tips
When assessing for a pneumothorax, perform a thorough scan of the chest wall in the location where air is most likely to be in the patient (Figure 1b). If there is visualization of any of the following findings, one can quickly and confidently rule out a pneumothorax: lung sliding, lung pulse, a single B-line, or the seashore sign using M-mode. Figure 3 is a flow chart on how to assess for a pneumothorax with lung ultrasonography.
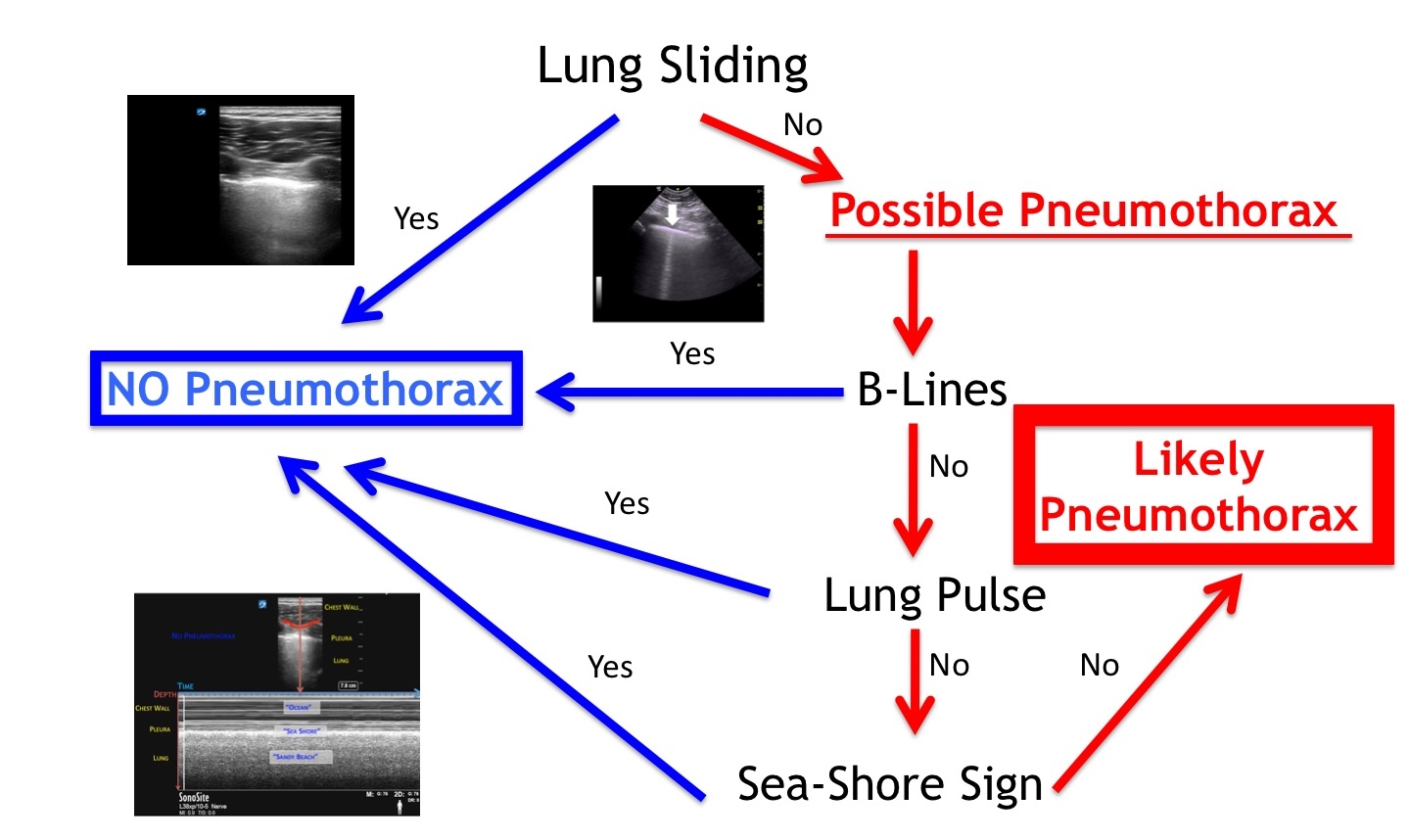
Figure 3: Pneumothorax flow chart
Clinical Case Revisited
You use the ultrasound machine and probe from the supraclavicular block to scan broadly on bilateral chest walls in between the second and third rib midclavicular line. You can clearly see lung sliding with a predominance of B-lines emerging from the pleura and “white-out” of the lung. You apply M-mode and see the seashore sign. You confidently rule out a pneumothorax, confirm your suspicion for a worsening CHF exacerbation, and treat accordingly. Watch a video demonstrating the lung ultrasonography and BLUE protocol.
References
- Ding W, Shen W, Yang J, He X, Zhang, M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest 2011;140(4):859–866. doi:10.1378/chest.10-2946.
- Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008;134(1):117–125. doi:10.1378/chest.07-2800.
Leave a commentOrder by
Newest on top Oldest on top