POCUS Spotlight: Point-of-Care Ultrasound in Cardiopulmonary Resuscitation
Cite as: Bughrara N, Panzer O, Pustavoitau A. POCUS spotlight: point-of-care ultrasound in cardiopulmonary rescusitation. ASRA Pain Medicine News 2022;47. https://doi.org/10.52211/asra0250122.016
Introduction
In-hospital cardiac arrest (IHCA) occurs in more than 290,000 adults annually in the United States.1 Non-shockable rhythms such as asystole and pulseless electrical activity (PEA) account for 81% of IHCA, with the most common causes being hypotension (50%-60%) and acute respiratory insufficiency (15%-40%).1 The American Heart Association Advanced Cardiac Life Support (ACLS) algorithm is the standard of care in managing IHCA. This algorithm emphasizes the performance of high-quality cardiopulmonary resuscitation (CPR) with minimizing pulse/ rhythm checks to fewer than 10 seconds. The ACLS guidelines also suggest that point-of-care ultrasound (POCUS) can be used to identify cardiac motion and potential reversible causes of PEA arrest, when an experienced sonographer is present and POCUS would not interfere with CPR.2,3
Detection of Cardiac Motion
Patients in PEA arrest who have coordinated electrical and myocardial activity visualized on POCUS (pseudo-PEA) have better prognosis compared to patients with no myocardial activity on POCUS (true PEA, or cardiac standstill) (Video 1). Recent meta-analysis demonstrated that spontaneous cardiac motion on ultrasound has a pooled sensitivity of 95% and specificity of 80% in predicting return of spontaneous circulation (ROSC).4 While ROSC is rare in patients with true PEA, ACLS guidelines recommend against using POCUS findings to terminate resuscitative efforts.5
Video 1. Subcostal 4-chamber view demonstrating cardiac standstill. RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle (click to view)
Potential Reversible Causes in Pseudo-PEA
Pericardial Tamponade
Pericardial tamponade is identified in 5%-22% of patient with PEA arrest.6,7 POCUS findings of pericardial tamponade include the presence of either small (acutely developing) or large (chronically developing) pericardial effusion, right atrial (RA) systolic collapse, right ventricular (RV) diastolic collapse, and plethoric inferior vena cava (IVC) (Videos 2 and 3). The diagnosis of tamponade using POCUS may be difficult in post-cardiac surgery patients who frequently present with a localized collection; transesophageal echocardiography (TEE) should then be used to make the diagnosis. A concomitant pleural effusion might potentially worsen the tamponade physiology. Therefore, imaging of the pleural space can be performed during chest compressions, with thoracentesis possibly reversing the tamponade physiology8 (Video 4).
Video 2. Subcostal 4-chaber view demonstrating pericardial effusion. RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle (click to view)
Supplemental Video 3. Subcostal IVC view demonstrating plethoric inferior vena cava (IVC) during chest compressions. RA, right atrium. (click to view)
Supplemental Video 4. Sonogram of the lateral inferior thorax demonstrating pleural effusion (click to view)
Pulmonary Embolism
Acute RV failure caused by pulmonary embolism (PE) is a common cause of cardiac arrest in up to 30% of patients with PEA arrest.9 Typical ultrasound findings include RV and RA dilatation on the four-chamber and septal flattening (so called D-sign) on the short-axis views. Also, RV systolic function is reduced in about half of the patients, as evidenced by a decreased tricuspid annular plane systolic excursion on the apical four-chamber view10 (Video 5). If color doppler is applied over the tricuspid valve, significant regurgitation is commonly found. While finding a thrombus in transit in the right heart chambers or within the pulmonary arteries is diagnostic of acute PE, it is rarely visualized and advanced imaging skills are required to locate thrombus.
Supplemental Video 5. Subcostal 4-chaber view demonstrating dilated and depressed right ventricular function. RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle (click to view)
The above findings (other than thrombus-in-transit) are not pathognomonic to PE as they are also seen during a pulmonary hypertensive crisis (PHTC). Chronic changes presenting as RV free-wall hypertrophy (thickness greater than 5 millimeters in diastole) and RA dilatation make a PHTC more likely, whereas a thin-walled RV free wall and a normal size RA point toward an acute process. Finally, chamber sizes should only be assessed in patients with preserved cardiac activity, as the RV can appear dilated in asystole independent of the etiology of cardiac arrest.11 If PE is suspected, the lower extremity venous ultrasound can be performed to evaluate for deep venous thrombosis. Evidence of a thrombus warrants a careful risk/benefit analysis to initiate empiric anticoagulation or thrombolytic therapy.
Myocardial Infarction
Myocardial ischemia is the most common cause of cardiac arrest with shockable rhythm. Presence of acute left ventricular (LV) dysfunction should raise the suspicion for acute coronary thrombosis as the cause of non-shockable PEA arrest and asystole (Video 6). Echocardiographic assessment of regional wall motion should be performed immediately after ROSC to minimize time to coronary revascularization.
Supplemental Video 6. Subcostal 4-chaber view demonstrating dilated and depressed left ventricular function. RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle (click to view)
Severe Hypovolemia
Small hyperdynamic LV that obliterates during systole is seen in cardiac arrest due to hypovolemia (Video 7).12 However, small collapsing LV size does not always correlate with intravascular volume status.11 In patients with trauma, a small (less than 1 cm), collapsible IVC predicts hemorrhagic shock responsive to volume loading (Video 8).12
Supplemental Video 7. Demonstrating underfilled heart. RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle (click to view)
Supplemental Video 8. Demonstrating small and collapsible inferior vena cava. RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle (click to view)
Tension Pneumothorax
Ultrasound diagnosis of pneumothorax is made by the absence of lung sliding (not pathognomonic) and B-lines in the appropriate clinical setting. Use of lung ultrasound can reduce time to needle decompression.13 During CPR, ultrasound can be used to assess for iatrogenic tension pneumothorax.
POCUS Protocols during CPR
During CPR, the use of POCUS remains a subject of investigation due to safety concerns. Its use was associated with prolonged pauses during pulse checks, which may reduce chances to achieve ROSC. However, prolonged pauses depend on sonographer experience. For example, when an attending or fellowship-trained practitioner acquires images, CPR is interrupted for shorter periods.14
To shorten the duration of interruptions in CPR, protocolized approaches to use ultrasound were developed.15-22 After the introduction of the protocols, the length of CPR interruptions uniformly decreases, although often not to below 10 seconds. With no evidence to support one approach over others, we would like to highlight the EASy-ALS protocol (outlined in Table 1, Figures 1 and 2, and Video 9). The EASy-ALS protocol is the protocol utilized by anesthesiology residents during IHCA.22 This protocol calls for prerequisite training including simulation, which focuses on teamwork, communication, high-quality CPR, and limiting pulse/rhythm checks to fewer than 10 seconds. In our experience, simulation-based training results in consistent shortening of interruptions in CPR during simulated cardiac arrest.23
Table 1. EASy-ALS protocol


Figure 1. Algorithm for the use of EASy-ALS. This algorithm incorporates FOCUS into the ≤10-s pulse/rhythm check of CPR. A systematic approach allows identification of a shockable rhythm if present and completion of EASy-ALS to search for a cardiac cause of the event without holding chest compressions for >10 s. (Used with permission from N. Bughrara, MD, Albany, NY.) CPR indicates cardiopulmonary resuscitation; EASy-ALS, echocardiographic assessment using subcostal-only view in advanced cardiac life support; FOCUS, focused cardiac ultrasound; PEA, pulseless electrical activity; ROSC, return of spontaneous circulation; RWMA, regional wall motion abnormality; VF, ventricular fibrillation; VT, ventricular tachycardia.
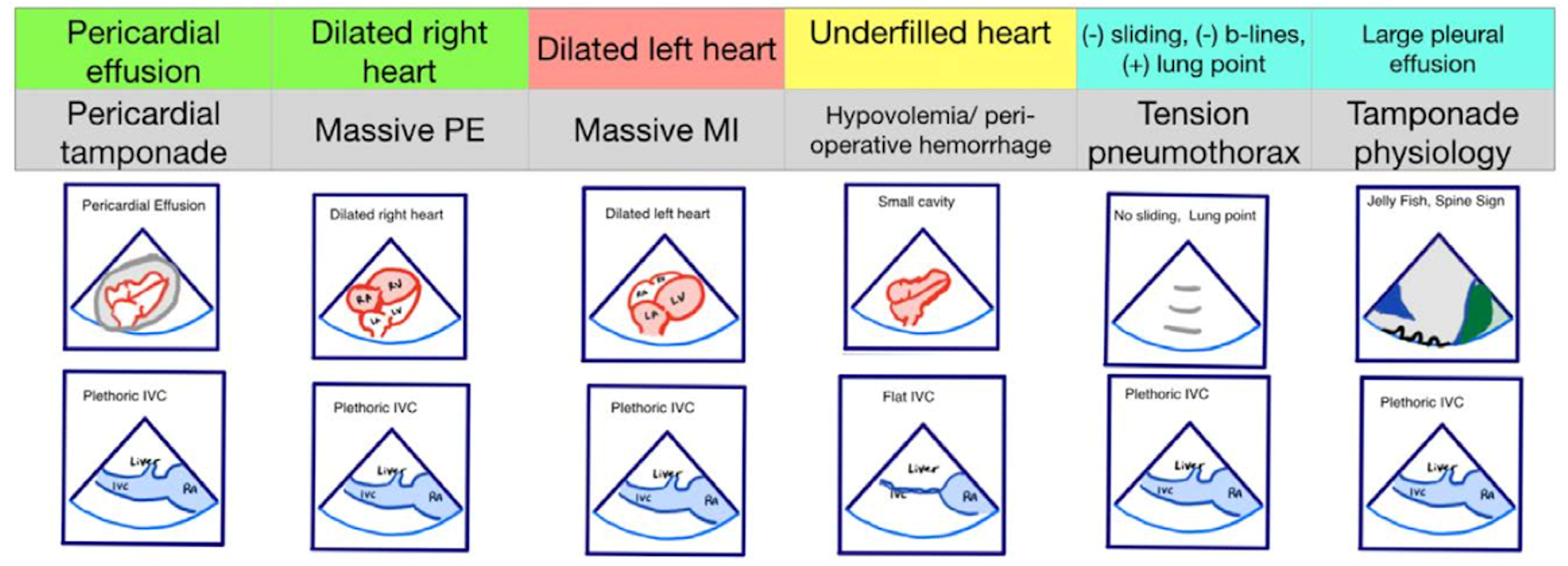
Figure 2. Pseudo-PEA phenotypes identified with EASy-ALS. The primary phenotypes to be identified with EASy-ALS are pericardial effusion, which may result in pericardial tamponade, dilated right heart, which pay occur with massive PE, dilated left heart, which may be seen with massive MI, and underfilled heart, which is associated with hypovolemia. Extracardiac views may be obtained as well; these include IVC ultrasound to assess volume status and RV filling pressure and lung ultrasound to assess for pleural effusion or tension pneumothorax. (Courtesy of N. Bughrara, MD, Albany, NY). EASy-ALS indicates echocardiographic assessment using subcostal-only view in advanced cardiac life support; IVC, inferior vena cava; MI, myocardial infarction; PE, pulmonary embolism; PEA, pulseless electrical activity; RA, right atrium; RV, right ventricular.
Supplemental Video 9. Demonstrating EASy-ALS in a simulated setting (click to view)
The responding resident is alerted to an acutely decompensating patient in the SICU or medical or surgical ward by direct call from the primary service or by overhead Code Blue page. On arrival, the resident prepares to serve as a sonographer and complete an EASy-ALS exam. The ultrasound probe is placed in the subcostal window before the pulse/rhythm check without obstructing chest compressions. The code leader, a senior primary care team provider, is responsible for holding and resuming chest compressions. The code leader assigns a nurse to count down 10 seconds during the pulse/rhythm check; this is standard at our institution. After resumption of chest compressions, the resident interprets recorded images and communicates findings to the code leader. The primary phenotypes (Figure 2) to be identified are cardiac standstill (see Video 1), pericardial effusion (Video 2), dilated right ventricle (RV) (Video 5), dilated left ventricle (LV) (Video 6), and underfilled heart (Video 7). The resident can obtain extracardiac views (such as the subcostal IVC View, Video 3), between pulse/rhythm checks.
Local Anesthetic Systemic Toxicity
Local anesthetic systemic toxicity (LAST) can occur during any type of regional anesthesia and can lead to cardiac arrest, particularly when high volumes of long-acting local anesthetics (e.g., bupivacaine) are injected. Early signs of LAST include perioral numbness, metallic taste in the mouth, and tinnitus. Seizures and hemodynamic instability may ensue. Tachycardia and hypertension are followed by bradycardia and hypotension, and eventually by ventricular arrhythmias and asystole. LAST symptoms typically develop within one minute, although a delayed onset after more than one hour of injection has been described.24
The role of POCUS during LAST is not well defined, but it could benefit the patient if cardiovascular collapse occurs late after LA injection. Expected findings include ventricular fibrillation or asystole, while other causes for hemodynamic instability can be detected as well.
During the resuscitation, POCUS may confirm either success (ie, reestablished synchronized cardiac activity) or failure (standstill) of therapy with lipid emulsion and trigger the initiation of cardiopulmonary bypass.
TEE in Cardiac Arrest Management
Obtaining surface ultrasound images can be limited by certain barriers like subcutaneous air or dressings in the areas of interest. In such cases, TEE can be used, although it is limited by its availability and the need for endotracheal intubation.25-26 TEE has the ability to not only assist with the diagnosis of reversible causes of cardiac arrest but also to guide optimal chest compressions, since in about 80% of patients the intrathoracic structures immediately under the point of compressions are the ascending aorta, the aortic root, or the LV outflow tract. Thus, the compression point may need to be shifted toward the xiphoid process for more effective ventricular compressions.27

Nibras Bughrara, MD, FCCM, FASA, is an associate professor of anesthesiology and surgery, the director of the Anesthesia Critical Care Division, and the director of Critical Care Echocardiography in the department of Anesthesiology and Critical Medicine at Albany Medical College in Albany, NY.

Oliver Panzer, MD, is an associate professor of anesthesiology, the director of Perioperative Ultrasound, and the co-director of the post-anesthesia care unit in the department of Anesthesiology and Critical Care Medicine at Columbia University Medical Center in New York, NY.

Aliaksei Pustavoitau, MD, MHS, FCCM, is an associate professor of Anesthesiology and Critical Care Medicine, the director of the Perioperative Ultrasound Program, the medical director of Respiratory Care Services and the director of the Hopkins Triage and Integration Physician Program in the department of Anesthesiology and Critical Care Medicine at Johns Hopkins School of Medicine in Baltimore, MD.
References
- Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In-hospital cardiac arrest: a review. JAMA 2019 Mar 26;321(12):1200-10. https://doi.org/10.1001/jama.2019.1696
- Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010;122:S729–S67. https://doi.org/10.1161/CIRCULATIONAHA.110.970988
- Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part II: cardiac ultrasonography. Crit Care Med 2016;44:1206–27. https://doi.org/10.1097/CCM.0000000000001847
- Blyth L, Atkinson P, Gadd K, et al. Bedside focused echocardiography as predictor of survival in cardiac arrest patients: a systematic review. Acad Emerg Med 2012;19:1119-26. https://doi.org/0.1111/j.1553-2712.2012.01456.x
- Panchal AR, Bartos JA, Cabañas JG, et al. Adult basic and advanced life support writing group. part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020;142(16 Suppl. 2):S366-S468. https://doi.org/10.1161/CIR.0000000000000916
- Breitkreutz R, Price S, Steiger HV, et al. Focused echocardiographic evaluation in life support and peri-resuscitation of emergency patients: a prospective trial. Resuscitation. 2010;81:1527-33. https://doi.org/10.1016/j.resuscitation.2010.07.013
- Chardoli M, Heidari F, Rabiee H, et al. Echocardiography integrated ACLS protocol versus conventional cardiopulmonary resuscitation in patients with pulseless electrical activity cardiac arrest. Chin J Traumatol 2012;15:284-7.
- Werlang ME, Pimentel MR, Diaz-Gomez JL. Thoracentesis-reverting cardiac tamponade physiology in a patient with myxedema coma and large pleural effusion. Proc (Bayl Univ Med Cent). 2017 Jul;30(3):295-7. https://doi.org/10.1080/08998280.2017.11929620
- Comess KA, DeRook FA, Russell ML, Tognazzi-Evans TA, Beach KW. The incidence of pulmonary embolism in unexplained sudden cardiac arrest with pulseless electrical activity. Am J Med 2000;109:351-6. https://doi.org/10.1016/s0002-9343(00)00511-8
- Bova C, Greco F, Misuraca G, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003;21:180-3. https://doi.org/10.1016/S0735-6757(02)42257-7
- Aagaard R, Løfgren B, Grøfte T, et al. Timing of focused cardiac ultrasound during advanced life support: a prospective clinical study. Resuscitation 2018;124:126–31. https://doi.org/10.1016/j.resuscitation.2017.12.012
- Yanagawa Y, Nishi K, Sakamoto T, et al. Early diagnosis of hypovolemic shock by sonographic measurement of inferior vena cava in trauma patients. J Trauma 2005;58:825-85. https://doi.org/10.1097/01.TA.0000145085.42116.A7
- Moore CL. Surgeon-performed ultrasound for pneumothorax in the trauma suite. J Trauma 2004;57:681-2. https://doi.org/10.1097/01.ta.0000141032.55574.6f
- Clattenburg EJ, Wroe P, Brown S, et al. Point-of-care ultrasound use in patients with cardiac arrest is associated with prolonged cardiopulmonary resuscitation pauses: a prospective cohort study. Resuscitation, 2018;122:65-8. https://doi.org/10.1016/j.resuscitation.2017.11.056
- Breitkreutz R, Uddin S, Steiger H, et al. Focused echocardiography entry level: new concept of a 1-day training course. Minerva Anestesiologica 2019;75(5):285-92.
- Testa A, Cibinel GA, Portale G, et al. The proposal of an integrated ultrasonographic approach into the ALS algorithm for cardiac arrest: the PEA protocol. Eur Rev Med Pharmacol Sci 2010;14:77-88.
- Hernandez C, Shuler K, Hannan H, Sonyika C, Likourezos A, Marshall J: CAUSE: Cardiac arrest ultra-sound exam: a better approach to managing patients in primary non-arrhythmogenic cardiac arrest. Resuscitation 2008;76:198-206. https://doi.org/10.1016/j.resuscitation.2007.06.033
- Gardner KF, Clattenburg EJ, Wroe P, et al. The Cardiac Arrest Sonographic Assessment (CASA) exam: a standardized approach to the use of ultrasound in PEA. Am J Emerg Med 2018;36:729-31. https://doi.org/10.1016/j.ajem.2017.08.052
- Lichtenstein D, Malbrain ML: Critical care ultrasound in cardiac arrest.Technological requirements for per- forming the SESAME-protocol: A holistic approach. Anaesthesiol Intensive Ther 2015;47:471-81. https://doi.org/10.5603/AIT.a2015.0072
- Niendorff DF, Rassias AJ, Palac R, et al. Rapid cardiac ultrasound of inpatients suffering PEA arrest performed by nonexpert sonographers. Resuscitation 2005;67:81-7. https://doi.org/10.1016/j.resuscitation.2005.04.007
- Breitkreutz R, Walcher F, Seeger FH. Focused echocardiographic evaluation in resuscitation management: concept of an advanced life support-conformed algorithm. Crit Care Med 2007;35(Suppl.5):S150-61. https://doi.org/
- Bughrara N, Herrick SL, Leimer E, Sirigaddi K, Roberts K, Pustavoitau A: Focused cardiac ultrasound and the peri-resuscitative period: A case series of resident-performed echocardiographic assessment using subcostal-only view in advanced life support.A A Pract 2020; 14:e01278. https://doi.org/10.1097/01.CCM.0000260626.23848.FC
- Cha SL, Gottschalk A, Su E, et al. Use of point-of-care ultrasonography in simulation-based advanced cardiac life support scenarios. J Anesth Perioper Med 2018;5:53-60.
- Gitman M, Fettiplace M, Weinberg G. Local anesthetic systemic toxicity. NYSORA. Available at: https://www.nysora.com/foundations-of-regional-anesthesia/complications/local-anesthetic-systemic-toxicity/. Accessed April 5, 2022.
- Fair J, Mallin M, Mallemat H, et al. Transesophageal echocardiography: guidelines for point-of-care applications in cardiac arrest resuscitation. Ann Emerg Med 2018;71:201-7. https://doi.org/10.1016/j.annemergmed.2017.09.003.
- Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Coll Cardiol 2007; 50:187-204. https://doi.org/10.1016/j.jacc.2007.05.003.
- Shin J, Rhee JE, Kim K. Is the inter-nipple line the correct hand position for effective chest compression in adult cardiopulmonary resuscitation? Resuscitation 2007; 75:305-10. https://doi.org/10.1016/j.resuscitation.2007.05.003.









Leave a commentOrder by
Newest on top Oldest on top