How I Do It: Restorative Neuromodulation for Chronic Low Back Pain
Cite as: Liao J, Leong MS. How I do it: restorative neuromodulation for chronic low back pain. ASRA Pain Medicine News 2025;50. https://doi.org/10.52211/asra110125.016.
How I Do It
Introduction
Chronic axial low back pain (LBP) is a prevalent and disabling condition worldwide.1 In a subset of patients, dysfunction or atrophy of the lumbar multifidi muscles contributes to persistent pain and/or segmental instability.2 The lumbar multifidi are essential for spinal stabilization, movement control, proprioception, nociception, core stability, and protection against lumbar injury.3-5 Dysfunction may be primary or secondary to chronic lumbar spondylosis, inactivity, deconditioning, prior denervation procedures, or surgical instrumentation.
Restorative neuromodulation of the lumbar multifidi is a specialized form of peripheral nerve stimulation designed to re-educate and re-engage low back musculature, thereby improving neuromuscular function and reducing pain.6 This is achieved via stimulation of the L2 dorsal ramus medial branches to re-engage the lumbar chain.
Conventional LBP treatments—physical therapy, medications, steroid injections, and radiofrequency ablation—are primarily palliative. Surgical options range from neuromodulation (spinal cord stimulation, dorsal root ganglion stimulation) to fusion procedures. Restorative peripheral nerve stimulation offers a rehabilitative approach that may reduce the need for repeat interventions or surgeries.
Currently, an implantable restorative neuromodulation system targeting lumbar multifidus dysfunction has been approved by the FDA.2 Unlike other peripheral nerve or spinal cord stimulator systems that use sensory stimulation to decrease the perception of pain, this system specifically uses motor stimulation to promote neuromuscular re-education and functional rehabilitation.2
This article describes our technique for bilateral lead placement and internal pulse generator (IPG) implantation under fluoroscopic guidance, with intraoperative motor testing to ensure optimal placement.
Procedural Goal
Activate the lumbar multifidus network by stimulating the L2 dorsal ramus medial branches using bilateral leads secured to the L2–3 intertransversarii muscles.
| Ideal Candidate |
| ≥ 6 months of axial LBP |
| MRI evidence of multifidus atrophy or dysfunction |
| Failure of conservative management (eg, physical therapy, medications) |
| No untreated radicular pain |
| Psychological suitability for neuromodulation |
Positive provocative physical exam findings:
|
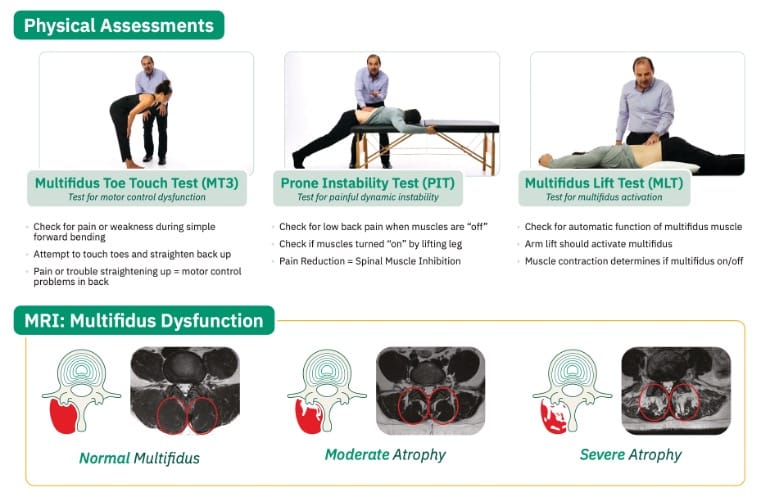
Image obtained from Mainstay Medical
Anesthetic and Positioning Considerations
The procedure is performed under monitored anesthesia care or general anesthesia. Intraoperatively, multifidus muscle contraction is tested. If paralytics were used for general anesthesia, then reversal agents for neuromuscular blockers should be available. Preoperative antibiotics must be given routinely as there are incisions involved.
The patient should be in the prone position with one or two pillows or a positioning device under the abdomen to reduce lumbar lordosis. The arms should be untucked and placed in “superman” position to optimize the lateral view on fluoroscopy.
| Recommended Tools | |
| Imaging |
|
| Local anesthetics |
|
| Intraoperative tools |
|
| Device kit |
|
| Irrigation |
|
| Closure |
|
| Fluoroscopic Views | |
| True Poster-Anterior (PA) View |
|
| True Lateral View |
|
| Ipsilateral Oblique View (optional) |
|
Step-by-Step Technique
- Obtain PA view centered at L3, visualizing L2 and L4.
- Place marker 22G or 25G spinal needles bilaterally at L3 TP–SAP junction; confirm tip at superior TP border on the lateral view.
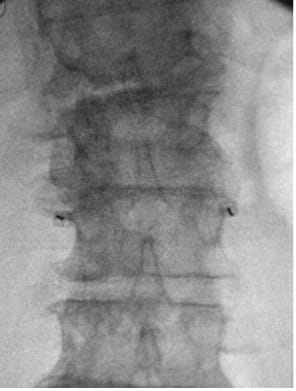
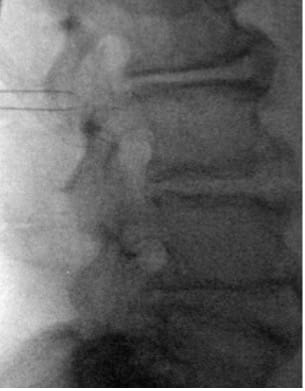
- Infiltrate L3–L4 interlaminar space; make 2–4 cm vertical midline incision just cephalad to L4 SP with cutdown to spinous process.
- Create a 3–4 cm diameter inferolateral tension-relief loop pocket via finger dissection towards the anticipated IPG site.
- In PA view, introduce guide needle just off midline from the superior L4 SP border towards the ipsilateral L3 TP–SAP junction marker; in lateral view, advance anteriorly to TP to traverse L2–L3 intertransversarii (~45°).
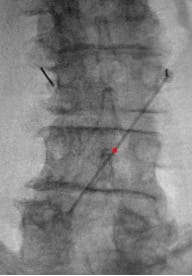
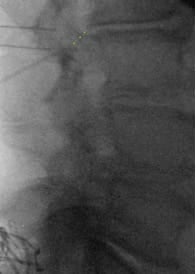
- In the lateral view, advance the guidewire towards the inferior dorsal L2 body; adjust depth if resistance is encountered. Prior to removing the guide needle and exchanging for the introducer sheath/dilator, use a #11 scalpel to make a stab incision along the guide needle trajectory adjacent to the L4 SP to create room for the introducer sheath/dilator.
- Advance introducer sheath/dilator over guidewire past TP; remove guidewire/dilator.
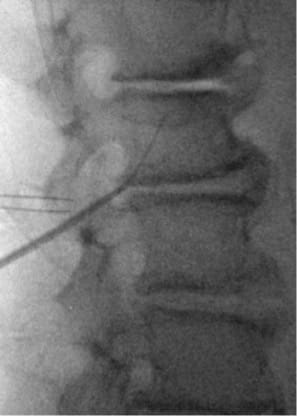
- Insert lead anterior to TP; retract introducer sheath to deploy lead and confirm tine engagement around intertransversarii via push–pull test under low-dose live fluoroscopy in lateral and AP view.
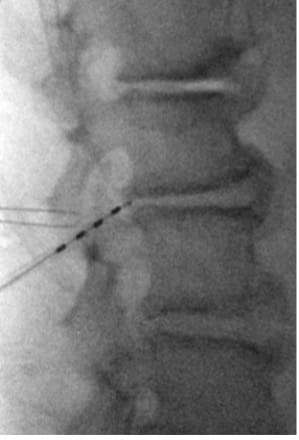
- Repeat on the contralateral side.
- Test impedances and motor responses; reverse paralytics if needed.
- Create an IPG pocket; tunnel leads from the inferolateral tension-relief loop pocket to the IPG pocket; test impedances and motor responses again with the IPG in the IPG pocket.
- Form tension-relief loops without sharp bends or significant friction points; tuck the loop into the tension-relief loop pocket.
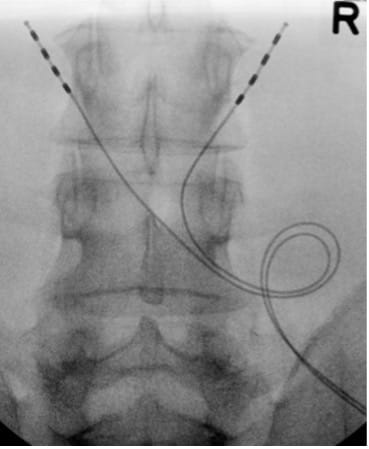
- Irrigate, close with layered sutures, and apply adhesives and dressing.
Post-operative Care
This is typically a same-day, outpatient surgical procedure. Postoperative antibiotics are usually recommended per Neurostimulation Appropriateness Consensus Committee guidelines.7
Best practices require a 1-week postoperative follow-up for a routine wound check, followed by a 2-week postoperative check when the device is turned on. The device is turned on at the 2-week postoperative appointment to optimize soft tissue recovery before activation of the multifidi muscles. The device company facilitates the programming to optimize muscular contraction at a duration and frequency tailored to individual patients.2,6
Recommended activity precautions focus on limiting rigorous activities that may result in lead migration, such as repetitive bending, lifting, and twisting, during the first 4 to 6 weeks. Furthermore, preventing moisture from entering the incision sites will reduce the risk of infection.
Outcomes
Longitudinal targeted multifidi activation via stimulation of the L2 medial branch nerves promotes neuromuscular re-education and rehabilitation of the lumbar spine. Restorative neuromodulation through this methodology decreases disability, increases functionality, and reduces chronic low back pain.


References
- Lucas JW, Connor EM, Bose J. Back, Lower limb, and upper limb pain among U.S. adults, NCHS Data Brief, No. 415, National Center for Health Statistics, 2021. https://doi.org/10.15620/cdc:107894
- Gilligan C, Volschenk W, Russo M, et al. Five-Year longitudinal follow-up of restorative neurostimulation shows durability of effectiveness in patients with refractory chronic low back pain associated with multifidus muscle dysfunction. Neuromodulation 2024;27(5):930-43. https://doi.org/10.1016/j.neurom.2024.01.006
- Ward SR, Eng CM, Gottschalk LJ, et al. The architectural design of the lumbar multifidus muscle supports its role as stabilizer. J Biomech 2006; 39:S101.
- Kim C, Gottschalk C, Eng SW, et al. The multifidus muscle is the strongest stabilizer of the lumbar spine. Spine J 2007;7(5):76S.
- Rosatelli AL, Ravichandiran K, Agur AM. Three-Dimensional study of the musculotendinous architecture of lumbar multifidus and its functional implications. Clin Anat 2008;21(6):539-46.https://doi.org/10.1002/ca.20659
- Gilligan C, Volschenk W, Russo M, et al. Long-Term outcomes of restorative neurostimulation in patients with refractory chronic low back pain secondary to multifidus dysfunction: two-year results of the ReActiv8-B pivotal trial. Neuromodulation 2023;26(1):87-97. https://doi.org/10.1016/j.neurom.2021.10.011
- Deer TR, Russo MA, Grider JS, et al. The Neurostimulation Appropriateness Consensus Committee (NACC): recommendations for surgical technique for spinal cord stimulation. Neuromodulation 2022;25(1):1-34. https://doi.org/10.1016/j.neurom.2021.10.015