ASRA Answers: Premedication Before Interventional Spine Procedures in Patients with Iodinated Contrast Allergy—Is It Necessary?
Cite as: Herrera T, Hunt C, Provenzano, D. ASRA answers: premedication before interventional spine procedures in patients with iodinated contrast allergy—is it necessary? ASRA Pain Medicine News 2026;51. https://doi.org/10.52211/asra020126.003.
ASRA Answers
Introduction
Iodinated contrast media (ICM) is essential for safe and accurate fluoroscopic-guided interventional spine procedures. The incidence of immediate hypersensitivity reactions (HSRs) has been significantly reduced by transitioning from ionic, high-osmolality contrast media (3.8%- 12.7%) to nonionic, low-osmolality contrast media (0.3%-1.4%).1,2 Despite the lower incidence, patients with a reported history of “contrast allergy” still present significant challenges and uncertainties for the treatment team. These cases prompt critical questions: Should the procedure proceed with premedication? Would it be safer to switch to an alternative contrast agent? Or should iodinated contrast be avoided altogether in favor of gadolinium or even ultrasound guidance?
Historically, recommendations across specialties have led to wide variation in practice; however, a more consistent framework has begun to emerge. In 2021, a multispecialty, multisociety practice advisory comprising 11 organizations published recommendations on radiographic contrast hypersensitivity reactions.3 Furthermore, in 2025, the American College of Radiology (ACR) and the American Academy of Allergy, Asthma & Immunology (AAAAI) issued a joint consensus statement recommending an approach that balances safety, feasibility, and evidence.2 The 2025 guidelines aim to standardize recommendations, particularly to address confusion about pretreatment and switching contrast media within the same class to reduce future ICM reactions.
| Severity | Findings | Vital Signs |
| Mild |
| Normal |
| Moderate |
| Normal |
| Severe |
| Altered (hypoxia, tachycardia, hypotension) |
Adapted from the 2025 ACR-AAAAI Consensus Statement.2
This article reviews the evidence for premedication, examines alternatives such as ICM substitution, dispels the myth of a shellfish allergy, and provides practical recommendations for interventional pain physicians.
Background
ICM Properties
Formerly, adverse reactions to ICM were classified into two broad categories: nonidiosyncratic and idiosyncratic anaphylactoid reactions. Nonidiosyncratic anaphylactoid reactions were further divided into chemotoxic and osmotoxic reactions. Now, immediate contrast reactions are termed either non-immunoglobulin E (IgE) mediated or IgE mediated. A significant risk factor for contrast reactions includes a previous contrast media hypersensitivity reaction. Other determinants include asthma, eczema, severe cardiovascular disease, female sex, and drug allergies. Documenting the severity of prior ICM reactions is crucial, as breakthrough reactions often follow the same pattern. ICM reactions should be reported in detail, including the volume and type of ICM used and the observed reaction.
Most adverse reactions to ICM are non-IgE-mediated anaphylactoid reactions, which result from direct activation of mast cells and basophils, leading to the release of histamine and other mediators.4 The osmolality and ionic nature of the contrast agent significantly influence the risk of these reactions. Ionic, high-osmolality contrast media exert stronger chemotoxic and osmotic effects, thereby promoting greater activation of inflammatory pathways and complement systems and increasing the likelihood of adverse reactions.4 In contrast, non-ionic low-osmolality agents are more physiologically inert, which produces fewer and less severe reactions.
Identifying and Grading Adverse Reactions
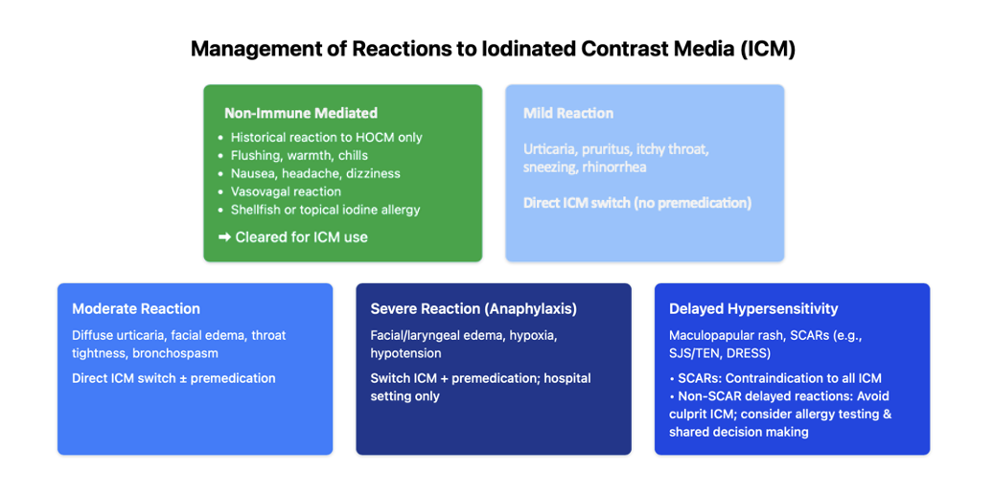
Adverse reactions to ICM are broadly categorized into mild, moderate, and severe immediate HSRs; delayed HSRs; and physiologic reactions. Immediate HSRs are defined as reactions occurring within 1 hour; however, most occur within 20 minutes. Delayed HSRs are Type IV hypersensitivity reactions that occur after 1 hour, but most often occur hours to days after ICM administration. Physiologic reactions can manifest as nausea, vomiting, headache, panic attacks, hypertension, chest pain, arrhythmias, and vasovagal reactions. These do not require premedication because they are non-immune-mediated. Accurate classification is critical for determining the treatment plan for future interventional procedures.
Evidence Review
When managing patients with a prior history of HSR to ICM, several strategies are commonly considered, including premedication with corticosteroids and/or antihistamines, ICM substitution, and switching to gadolinium.
Premedication and ICM Substitution
Premedication with corticosteroids and/or anti-histamines has traditionally been recommended for patients with a prior HSR to ICM. Most of the data supporting premedication comes from older studies involving ionic, high-osmolality agents, limiting their applicability in the era of non-ionic, low-osmolality contrast media.5 More recent studies demonstrate that premedication may offer limited protective benefit, with breakthrough reactions occurring in up to 2.1% of cases and often presenting with severity similar to the initial reaction.6 McDonald et al. reported that premedication with the same ICM yielded breakthrough reactions in 19% of patients. In contrast, a direct switch to a different low-osmolality ICM was markedly more effective, reducing breakthrough rates to 3% with premedication and 3% without.7 Similarly, Park et al. showed that ICM substitution to another low-osmolality contrast media (eg, iohexol, iopamidol, ioversol, iopromide, iobitridol, and iomeprol) reduced breakthrough HSR by 67.1%.8 Overall, evidence increasingly favors ICM substitution over routine premedication as the more effective strategy, highlighting the importance of accurate documentation of the culprit agent to guide future management.
The 2025 ACR–AAAAI consensus statement has provided further clarity on the approach to managing prior HSRs. For mild reactions (eg, localized urticaria, pruritus, or nasal congestion), premedication is not recommended. If the culprit ICM is known, substitution with an alternative agent is advised; if unknown, the formulary ICM should be administered. This is a departure from the previous American College of Radiology Contrast Manual, which recommended premedication to prevent hypersensitivity reactions. Additionally, premedication is not required for previous vasovagal responses or reactions to topical povidone-iodine. In cases of moderate reactions (eg, diffuse urticaria, facial edema, throat tightness, wheezing, bronchospasm), ICM substitution is recommended, while premedication may be considered on a case-by-case basis with shared decision-making. If the culprit agent is unknown, the formulary ICM should be administered with consideration of premedication. For severe prior reactions (eg, anaphylaxis; facial or laryngeal edema causing hypoxia; wheezing or bronchospasm with hypoxia; hypotension), both substitution and premedication are recommended with consideration of an alternative imaging modality. When the culprit agent is unknown, refer for skin testing and proceed with a negatively tested ICM in addition to premedication. In cases of previous severe reactions to ICM, the balance between the benefits and risks of the interventional pain procedure requiring the ICM needs to be strongly considered. Additionally, future procedures with ICM should be performed in the hospital setting with a rapid response team that has the ability to treat anaphylaxis. Lastly, severe cutaneous adverse reactions, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms, are contraindications to ICM use.2
If Premedication is Needed, What’s the Protocol?
Standard Oral Regimens
Traditional regimens include oral prednisone (50 mg at 13, 7, and 1 hour prior) or methylprednisolone (32 mg at 12 and 2 hours prior), with or without diphenhydramine.2
Accelerated IV Premedication
Accelerated IV premedication includes methylprednisolone sodium succinate 40 mg IV or hydrocortisone sodium succinate 200 mg IV immediately and then every 4 hours until contrast medium administration with diphenhydramine 50 mg IV 1 hour before contrast medium administration. 2
Role of Gadolinium in Interventional Spine Procedures
For years, gadolinium-based contrast media (GBCM) have been employed as substitutes in patients with ICM allergies. However, evidence has revealed substantial risks when these agents are used in neuraxial procedures including nephrogenic systemic fibrosis in patients with renal impairment, long-term brain deposition/retention, and encephalopathy/death after unintended intrathecal injection.9-12
As a result, gadolinium should not be used in epidural, intrathecal, or transforaminal injections.9-12 For non-neuraxial interventions, such as facet or peripheral joint injections, limited-dose GBCM may still be considered in rare cases when iodinated agents are unsuitable and no safe alternative exists.9
Dispelling the “Shellfish Allergy” and “Iodine Allergy” Myth
Many patients have documented “shellfish” and “iodine” allergies, which give the treatment team pause before proceeding with ICM administration. The origin of the shellfish allergy=iodine allergy=ICM allergy can be traced back to the poor understanding of the underlying causes of ICM and shellfish allergies and the coincidental overlap between two relatively common allergens. Iodine is an essential element and cannot trigger allergic reactions; no one is “allergic to iodine.” Most adverse reactions to ICM are caused by an anaphylactoid reaction related to osmolality, as described above. Adverse reactions to shellfish are actually associated with the protein, tropomyosin, not iodine. Similarly, responses to Betadine (povidone-iodine) are attributable to povidone-induced dermatitis, not to iodine. Overall, patients with shellfish allergy are at no greater risk for contrast reactions than the general population.
Conclusion and Recommendations
Practical Guidance
- Mild prior reaction:
o Known culprit ICM: No premedication; recommend ICM substitution.
o Unknown culprit ICM: No premedication; use ICM on formulary.
- Moderate prior reaction:
o Known culprit ICM: ICM substitution ± premedication.
o Unknown culprit ICM: Use ICM on formulary ± premedication.
- Severe prior reaction:
o Known culprit ICM: ICM substitution + premedication; perform procedure in a hospital setting with resuscitation resources.
o Unknown culprit ICM: Referral to allergy for skin testing. Use negatively tested ICM + premedication.
- Gadolinium: Avoid GBCAs in neuraxial injections due to risk of catastrophic neurotoxicity.
In summary, premedication cannot reliably prevent all breakthrough reactions. Instead, management should focus on obtaining a detailed history of prior reactions, substituting contrast agents when feasible, and considering premedication for patients with moderate HSRs. For patients with severe hypersensitivity, shared decision-making is essential. If using iodinated contrast media, ensure that all safety protocols are followed and perform the procedure in a hospital with rapid-response support. For interventional pain physicians, these principles can reduce unnecessary risk while maintaining procedural safety. In addition, gadolinium should not be considered a viable alternative for neuraxial injections because of the risk of severe neurotoxicity with inadvertent intrathecal administration.



References
- Kim SH, Park HJ, Kim HY, et al. Allergic-like reactions to iodinated contrast media: prevalence, risk factors and outcome in 456,930 patients. Br J Radiol 2014;87(1041):20140311. https://doi.org/10.1259/bjr.20140311
- Wang CL, Greenberger PA, Lieber MR, et al. ACR-AAAAI consensus statement on hypersensitivity reactions to iodinated contrast media. American College of Radiology and American Academy of Allergy, Asthma & Immunology; 2025.
- Dillman JR, Ellis JH, Cohan RH, et al. A multidisciplinary consensus guideline for the prevention and management of acute reactions to iodinated contrast media. Radiology 2021;298(2):E303-12. https://doi.org/10.1148/radiol.2020202147
- Brockow K, Christiansen C, Kanny G, et al. Management of hypersensitivity reactions to iodinated contrast media. Allergy 2021;76(3):737-47. https://doi.org/10.1111/all.14576
- Greenberger PA, Patterson R. The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J Allergy Clin Immunol 1991;87(4):867-72. https://doi.org/10.1016/0091-6749(91)90358-L
- Davenport MS, Dillman JR, Cohan RH, et al. Repeat iodinated contrast media administration in patients with prior reactions: frequency and severity of recurrent reactions. Radiology. 2009;253(2):372-9. https://doi.org/10.1148/radiol.2532082363
- McDonald JS, Hunt CH, Kallmes DF, et al. Strategies for reducing the risk of recurrent hypersensitivity reactions to iodinated contrast media. Radiology 2021;298(3):658-64. https://doi.org/10.1148/radiol.2021204494
- Park HJ, Kim KW, Lee SJ, et al. Re-exposure to iodinated contrast media in patients with prior moderate-to-severe hypersensitivity: effectiveness of premedication and risk factors for recurrent reactions. Radiology 2016;278(3):764-71. https://doi.org/10.1148/radiol.2015150917
- Benzon HT, Maus TP, Kang HR, et al. The use of gadolinium-based contrast agents in interventional spine procedures: safety, risks, and recommendations. Reg Anesth Pain Med 2021;46(7):580-8. https://doi.org/10.1136/rapm-2020-101629
- Provenzano DA, Stanos S, Jarrett P, et al. Gadolinium as a contrast agent in interventional pain procedures: risks and recommendations. Pain Physician 2019;22(4):E321–31.
- Hallo-Carrasco A, et al. Neurotoxic outcomes from intrathecal gadolinium: a systematic review. Neuroradiology 2024;66(1):17-25. https://doi.org/10.1007/s00234-023-03208-1
- Kohan L, Manchikanti L, Bhatia A, et al. Case report: catastrophic neurological outcomes after intrathecal gadolinium injection. Pain Physician 2022;25(1):E137-42.