How I Do It: Basivertebral Nerve Ablation for Vertebrogenic Pain
Cite as: Salmasi V, Sheth SJ, Naidu RK, et al. How I do it: basivertebral nerve ablation for vertebrogenic pain. ASRA Pain Medicine News 2025;50. https://doi.org/10.52211/asra110125.015.
How I Do It
Introduction
Chronic low back pain affects over 30 million Americans annually, with up to 80% of cases classified as “non-specific.”1,2 Vertebrogenic pain, a distinct syndrome caused by degenerative changes of vertebral endplates, represents a treatable clinical entity causing low back pain. Basivertebral nerve ablation (BVNA) offers targeted treatment with Level I evidence demonstrating sustained improvements persisting over 5 years.3,4
What is Vertebrogenic Pain?
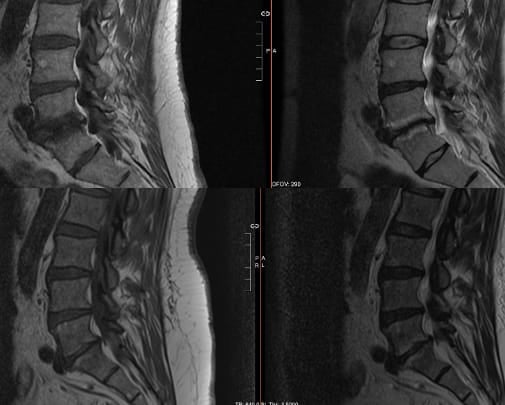
Vertebrogenic pain results from degenerative changes of vertebral endplates—the interface between intervertebral discs and vertebral bodies. Patients often describe deep, midline lumbar pain worsening with loading of the anterior spinal column: spinal flexion, prolonged sitting, bending, and lifting. Unlike facet-mediated pain, vertebrogenic pain is typically not influenced by lumbar spinal extension positions. The pathophysiology of this condition includes inflammatory and fibro-fatty changes, manifesting on MRI as modic changes: Type 1 (active inflammation, hypointense T1/hyperintense T2), Type 2 (fatty infiltration, hyperintense both sequences) (Figure 1).1,5-7 Neoinnervation of the vertebral endplates occurs via the basivertebral nerve; 90% of damaged endplates with Type 1 or Type 2 Modic changes demonstrate pathologic neural ingrowth compared to only 30% of annular tears. Immunohistochemical studies demonstrate the presence of substance P and protein gene product 9.5 within basivertebral nerve fibers, confirming their nociceptive function.1,5,8,9
How to Identify Patients
Patient selection for BVNA requires a systematic evaluation that combines clinical presentation, physical examination, imaging findings, and potentially, diagnostic injections to exclude other potential pain sources. Appropriate candidates present with chronic axial low back pain of at least 6 months’ duration, refractory to conservative management.1,3,10
Clinical history should reveal midline lumbar pain worsening with flexion-based activities (loading of the anterior column) with significant functional impairment. Extension-based pain can include vertebrogenic pain, but the differential is wider, inclusive of facetogenic pain and sacroiliac joint dysfunction. The absence of radicular symptoms, motor deficits, or sensory changes distinguishes vertebrogenic pain from nerve root compression. The diagnostic cornerstone is MRI demonstration of Type 1 and/or Type 2 Modic.1,3,10
Thermal BVNA is an FDA-approved treatment for these patients with vertebrogenic pain from L3-S1.1 Relative contraindications include active infections, pregnancy, skeletal immaturity, severe cardiopulmonary compromise, and active implantable pulse generators.10
Technical Procedure Details
A safe and technically successful BVNA procedure depends on meticulous planning and precise execution. The basivertebral nerve enters the vertebral body through the midline basivertebral foramen, traveling anteriorly 30%-50% of the sagittal diameter of the vertebral body, and then arborizes to innervate both the superior and inferior vertebral endplates.
Pre-Procedural Planning
A comprehensive imaging review is essential. Careful measurement of pedicle diameter is crucial as pedicles become progressively smaller at rostral spinal levels. Pedicle width should be at least 4.4 mm to safely accommodate the current instrumentation of the BVNA system. CT imaging provides superior bony detail and should be strongly considered for challenging anatomy, previous spinal surgery, planned extra-pedicular access, and levels above L3 (off-label treatment).
Critical Imaging Requirements
Obtaining true anteroposterior (AP) and lateral fluoroscopic views is fundamental to the technical success of the procedure. At the level of interest, the AP view must demonstrate symmetric pedicles with a clear "owl's eyes" appearance by tilting the bed left or right, rather than rotating the fluoroscope into an oblique position. The superior endplate should be aligned for proper AP visualization of the pedicles using cephalad or caudal tilt of the fluoroscope. At the level of interest, the lateral view should show superimposed pedicles with clear inferior and superior margins as well as clear vertebral body boundaries, which are optimized using fluoroscope wig-wag.
Procedural Technique
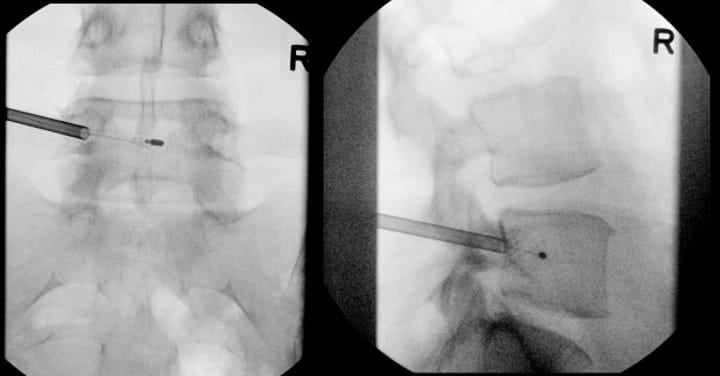
The transpedicular approach targets the basivertebral foramen in the AP-plane midline of the posterior aspect of the vertebral body. The trajectory must remain within the superior and inferior pedicle cortices. The site of docking on the periosteum for transpedicular access is typically at the radiographic junction of the superior articular process and transverse process.
Monitored anesthetic care is the most common type of anesthesia performed, although general anesthesia is an option as well. Local anesthesia is administered, followed by careful trocar advancement under fluoroscopic guidance. It is very important to check AP and lateral images to ensure that the trocar does not violate the medial border of the pedicle on the AP image prior to breaching the posterior wall of the vertebral body on lateral imaging. The trocar passes through the lateral pedicle aspect, staying superior to inferior cortex and lateral to medial cortex. Once the posterior vertebral body cortex is breached, the curved cannula assembly is advanced toward the stem of the basivertebral nerve at a point located 30%-50% of the sagittal diameter of the vertebral body (closer to the posterior wall). This allows for radiofrequency probe positioning proximal to the location of basivertebral nerve arborization. Final probe positioning varies by vertebral level to optimize targeting of the basivertebral nerve terminus. For the L3, L4, and L5 levels, the probe should be positioned at the midpoint between the superior and inferior endplates (approximately 50% of the cephalo-caudal diameter of the vertebral body) and 30-50% of the vertebral body width from the posterior wall (Figure 2). However, S1 vertebral anatomy requires modified positioning due to the unique sacral morphology. At the S1 vertebral body, the probe should be positioned closer to 50% of the sagittal diameter from the posterior wall and approximately 40% of the distance from the superior endplate to the inferior endplate. Proper positioning at each level is confirmed through multiple fluoroscopic views in both AP and lateral projections before initiating the ablation cycle.
Vertebrogenic pain results from degenerative changes of vertebral endplates—the interface between intervertebral discs and vertebral bodies.
Out-of-Box Thinking for Challenging Anatomy
Complex anatomical variations and surgical interventions require creative problem-solving and modified approaches.
Multi-Level Considerations
Patients with extensive modic changes across multiple levels may benefit from multi-level ablation, either staged or simultaneous, based on patient factors and insurance reimbursement requirements.
Off-Label Treatment Above L3
While FDA approval covers the L3-S1 levels, select cases may warrant treatment of higher levels with modic changes and a high level of confidence that the motion segment is a source of pain. Treatment above L3 requires exceptional care due to the progressively smaller pedicles and proximity of the neural structures (Figures 3 and 4).
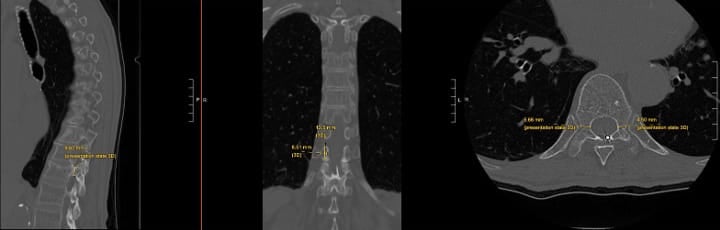
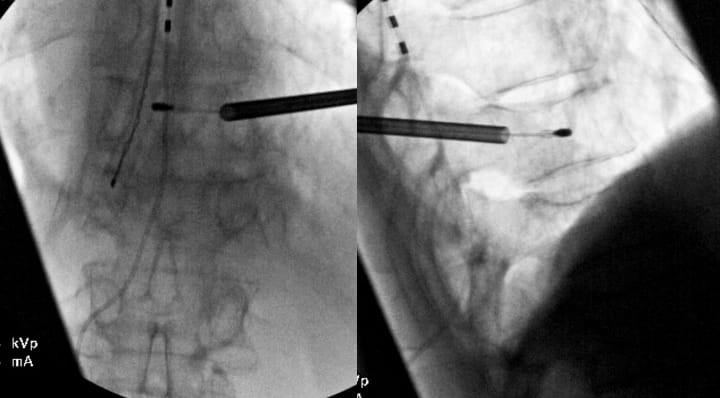
Critical considerations include (1) mandatory pedicle diameter measurement, (2) CT scan for pre-procedure planning is strongly recommended, (3) modified trajectory angles (ie parapedicular or extrapedicular approaches) to accommodate anatomy, (4) increased vigilance for neural structure proximity, and (5) potential for vascular trespass if an extrapedicular approach is being considered (segmental arteries).
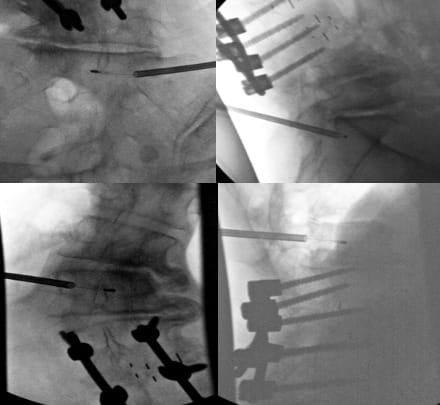
Scoliosis and Spinal Deformity
Patients with scoliosis present unique challenges due to sagittal plane deformity but also potentially rotational deformities and altered vertebral anatomy. The rotational component displaces pedicles from normal positions, requiring careful three-dimensional anatomy analysis before the procedure. Pre-procedural CT with multiplanar reconstructions is helpful for trajectory planning. The convex side often provides less challenging access due to pedicle orientation. To obtain accurate (AP) and lateral views, it is more effective to rotate the bed rather than the C-arm. This approach facilitates the acquisition of true lateral images, particularly when utilizing C-arm equipment that has a limited rotation capability of 90 degrees (Figure 5).
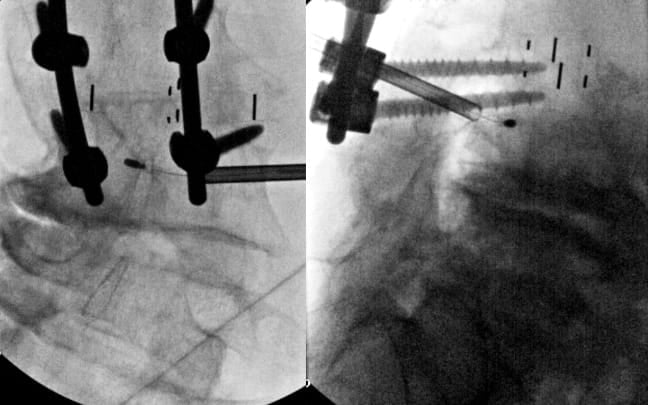
Patients with Existing Pedicle Screws
When pedicle screws occupy the standard transpedicular corridor, alternative approaches must be considered. In select cases where a CT scan demonstrates adequate space, a parapedicular approach above or below the pedicle screw can be considered while maintaining a safe distance from neural structures. This requires careful pre-procedural CT analysis to ensure a sufficient bone corridor and adequate distance from the traversing nerve root. Trajectory planning must account for the altered angles required to reach the basivertebral nerve while avoiding hardware collision. If there is inadequate space above and below the pedicle screw, an extrapedicular approach may be considered after careful examination of soft tissue and vascular anatomy (segmental artery in particular) adjacent to the pedicle root and vertebral body (Figure 6).
Clinical Efficacy, Effectiveness and Safety
BVNA efficacy and effectiveness has been established through robust clinical evidence, including a number of European and United States trials.4,12-17 A 5-year pooled analysis of 249 participants demonstrated a mean pain improvement of 4.32±2.45 points on the visual analog scale (p<0.0001) and a mean functional improvement of 28.0±17.5 point on the Oswestry Disability Index points (p<0.0001). Notably, 32.1% of participants reported complete pain relief, 72.7% reported condition improvement, and 68.7% resumed pre-pain activity levels.3
Healthcare utilization reductions included a 65.2% rate of opioid cessation of those participants consuming opioids pre-BVNA and a 58.1% reduction in spinal injections within 5 years post-BVNA. The safety profile of BVNA remains excellent with no serious device-related adverse events reported during long-term follow-up.3,18 Rare cases of vertebral compression fractures are reported in patients with preexisting osteoporosis. The decision to perform BVNA in patients with osteoporosis should be individualized as this is a rare potential complication, and the natural course of these patients without BVNA treatment is uncertain.19,20
Conclusion
BVNA represents a significant advancement in vertebrogenic pain management, offering targeted treatment with demonstrated durability of 5 years. Technical success of this procedure requires meticulous pre-procedural planning, optimized imaging, and adaptability in cases of challenging anatomy. The favorable safety profile and meaningful improvements establish BVNA as a valuable addition to the field of interventional pain management.




References
- Conger A, Smuck M, Truumees E, et al. Vertebrogenic pain: a paradigm shift in diagnosis and treatment of axial low back pain. Pain Med 2022; 23:S63-71. https://doi.org/10.1093/pm/pnac081
- Dillane JB, Fry J, Kalton G. Acute back syndrome—a study from general practice. Br Med J 1966; 2:82-4. https://doi.org/10.1136/bmj.2.5505.82
- Khalil JG, Truumees E, Macadaeg K, et al. Intraosseous basivertebral nerve ablation: a 5-year pooled analysis from three prospective clinical trials. Interv Pain Med 2024;3:100529.https://doi.org/10.1016/j.inpm.2024.100529
- Fischgrund JS, Rhyne A, Macadaeg K, et al. Long-term outcomes following intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 5-year treatment arm results from a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J 2020;29:1925-34. https://doi.org/10.1007/s00586-020-06448-x
- Fields AJ, Liebenberg EC, Lotz JC. Innervation of pathologies in the lumbar vertebral endplate and intervertebral disc. Spine J 2014;14:513-21. https://doi.org/10.1016/j.spinee.2013.06.075
- Lotz JC, Fields AJ, Liebenberg EC: The role of the vertebral end plate in low back pain. Global Spine J 2013; 3:153-64. https://doi.org/10.1055/s-0033-1347298
- Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 1988;166:193-9. https://doi.org/10.1148/radiology.166.1.3336678
- Bailey JF, Liebenberg E, Degmetich S, et al. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. J Anat 2011; 218:263-70. https://doi.org/10.1111/j.1469-7580.2010.01332.x
- Fras C, Kravetz P, Mody DR, et al. Substance P-containing nerves within the human vertebral body. an immunohistochemical study of the basivertebral nerve. Spine J 2003;3:63-7. https://doi.org/10.1016/s1529-9430(02)00455-2
- Sayed D, Naidu RK, Patel KV, et al.: Best practice guidelines on the diagnosis and treatment of vertebrogenic pain with basivertebral nerve ablation from the American Society of Pain and Neuroscience. J Pain Res 2022;15:2801-19. https://doi.org/10.2147/JPR.S378544
- Pierce AD. Intracept intraosseous nerve ablation system FDA approval. https://www.accessdata.fda.gov/cdrh_docs/pdf21/K213836.pdf. Published March 11, 2022. Accessed July 28, 2025.
- Smuck M, Truumees E, Macadaeg K, et al. Intraosseous basivertebral nerve ablation: Pooled long-term outcomes from two prospective clinical trials. Interv Pain Med 2023;2:100256.https://doi.org/10.1016/j.inpm.2023.100256
- Macadaeg K, Truumees E, Boody B, et al. A prospective, single arm study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-month results. N Am Spine Soc J 2020;3:100030. https://doi.org/10.1016/j.xnsj.2020.100030
- Becker S, Hadjipavlou A, Heggeness MH. Ablation of the basivertebral nerve for treatment of back pain: a clinical study. Spine J 2017;17:218-23. https://doi.org/10.1016/j.spinee.2016.08.032
- Boody BS, Sperry BP, Harper K, et al. The relationship between patient demographic and clinical characteristics and successful treatment outcomes after basivertebral nerve radiofrequency ablation: a pooled cohort study of three prospective clinical trials. Pain Med 2022;23:S2-13. https://doi.org/10.1093/pm/pnac050
- McCormick ZL, Sperry BP, Boody BS, et al. Pain location and exacerbating activities associated with treatment success following basivertebral nerve ablation: an aggregated cohort study of multicenter prospective clinical trial data. Pain Med 2022;23:S14-33. https://doi.org/10.1093/pm/pnac069
- Conger A, Burnham TR, Clark T, et al. The effectiveness of intraosseous basivertebral nerve radiofrequency ablation for the treatment of vertebrogenic low back pain: an updated systematic review with single-arm meta-analysis. Pain Med 2022;23:S50-62. https://doi.org/10.1093/pm/pnac070
- Smuck M, McCormick ZL, Gilligan C, et al. A cost-effectiveness analysis of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Spine J 2025;25:201-10. https://doi.org/10.1016/j.spinee.2024.09.016
- Fogel G, Dickinson J, Vuong S. Elective spinal transpedicular ablation of the basivertebral nerve of the vertebral segment in adult spinal deformity patients. Int J Spine Surg 2024;18:694-704. https://doi.org/10.14444/8632
- Fogel G, Musie J, Phillips TR, et al. Assessment and management of patients developing low energy vertebral compression fractures following basivertebral nerve ablation. Pain Med2024;25:249-51. https://doi.org/10.1093/pm/pnad132