POCUS Spotlight: Assessment of Right Ventricle with Echocardiography
Cite as: Gandhi Z, Siuba M, Dugar S. POCUS spotlight: assessment of right ventricle with echocardiography. ASRA Pain Medicine News 2024;49. https://doi.org/10.52211/asra110124.011.
Introduction
Historically, most research investigating cardiac function in health and disease focused on the left ventricle (LV). The right ventricle (RV) was merely considered a conduit transferring blood from venous to low-pressure pulmonary circulation. This has led to many people calling the right ventricle “the forgotten ventricle.” The use of pulmonary artery catheterization and now echocardiography, both comprehensive and point of care ultrasound (POCUS), has heralded an enhanced understanding of the crucial role and high prevalence of RV injury in critically ill patients.1,2 The occurrence of RV injury in sepsis, pulmonary embolism, acute respiratory distress syndrome, and RV myocardial infarction has been shown to be associated with higher morbidity and mortality.3-6 A comprehensive assessment of the RV remains challenging due to its retrosternal positioning, crescentic shape, and absence of echocardiographic landmarks.7,8 In addition, there is still a lack of standardized methods to assess and grade right ventricular function.9 This concise review summarizes basic RV assessment using POCUS.
Echocardiographic Views
The crescentic shape of the RV wrapping around the left ventricle in a U-shaped manner implies that no single echocardiographic view completely encompasses the entire RV. Hence, a comprehensive echocardiographic assessment of the RV requires multiple windows. These include the conventional apical four-chamber (A4C), parasternal long-axis (PLAX) and short-axis (PSAX), RV inflow and subcostal views, and RV-focused view (Figure 1). On a transesophageal echocardiogram, the mid-esophageal four-chamber, RV inflow-outflow views, and the trans-gastric short- and long-axis views are utilized for RV assessment. Most RV dimensions and measurements are obtained in A4C, making it one of the most important views. A conventional A4C view is obtained from the cardiac apex to place the LV in the center of the screen. In this view, the RV-free wall may be obscured (Video 1). The RV-focused view is obtained by lateral displacement of the transducer to obtain the largest RV dimensions in the long axis (Video 2).7,8,10,11 Dimensions obtained in the RV-focused view significantly improve the accuracy of RV measurements.12 This is especially important in critically ill patients, where these measurements may be used dynamically to ascertain trends and responses to interventions. In patients on positive pressure ventilation, the parasternal and apical views may be difficult to obtain. One should be well-versed in utilizing the subcostal view to get a more reliable assessment of the RV in this patient population (Video 3).13
This concise review summarizes basic RV assessment using POCUS.
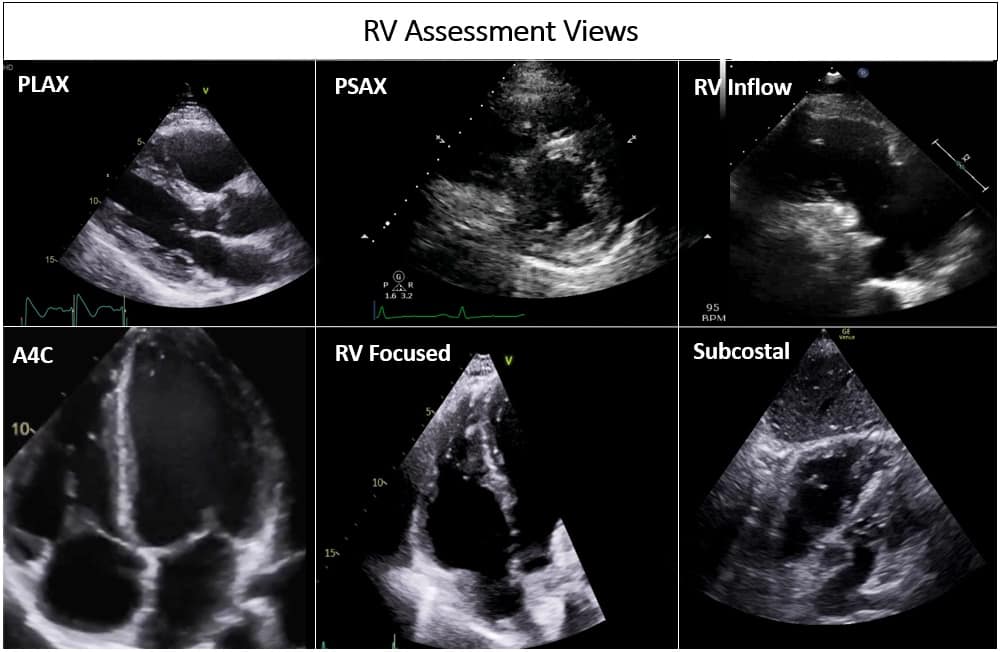
Figure 1: Different views assessing the right ventricle in transthoracic echocardiogram.
PLAX = parasternal long axis, PSAX = parasternal short axis, RV = right ventricle, A4C = apical 4 chamber
RV Dimensions
RV assessment can be qualitative or quantitative with attention to RV size, systolic function, pulmonary hemodynamics, or a combination thereof. The visual assessment of RV size and function begins in PLAX, using the “rule of thirds” to uncover the presence of RV dilation (Figure 2). In A4C or the RV-focused view, qualitatively, RV size >2/3 of LV size is considered dilated.7,8 Quantitatively, RV enlargement is present when the RV basal diameter is > 41 mm at the point of the tricuspid valve (TV) insertion to the RV free wall (RVFW) or RV mid-cavity dimension is >35 mm at the level of the open anterior TV leaflet on RVFW.10,11 A RV end-diastolic area to left ventricular end-diastolic area (RVEDA: LVEDA) >0.6 defines moderate RV dilation, while area of (RVEDA: LVEDA) ≥1 suggests severe RV dilation or acute cor-pulmonale, especially when septal dyskinesia is present.10, 12 McConnell’s sign is another qualitative sign of acute cor-pulmonale characterized by dyskinesia of the RVFW with relative sparring of the RV apex (Video 4). It is important to note that the reliability of measurements to assess RV size and function increases with further training and practice.13 (Figure 3)
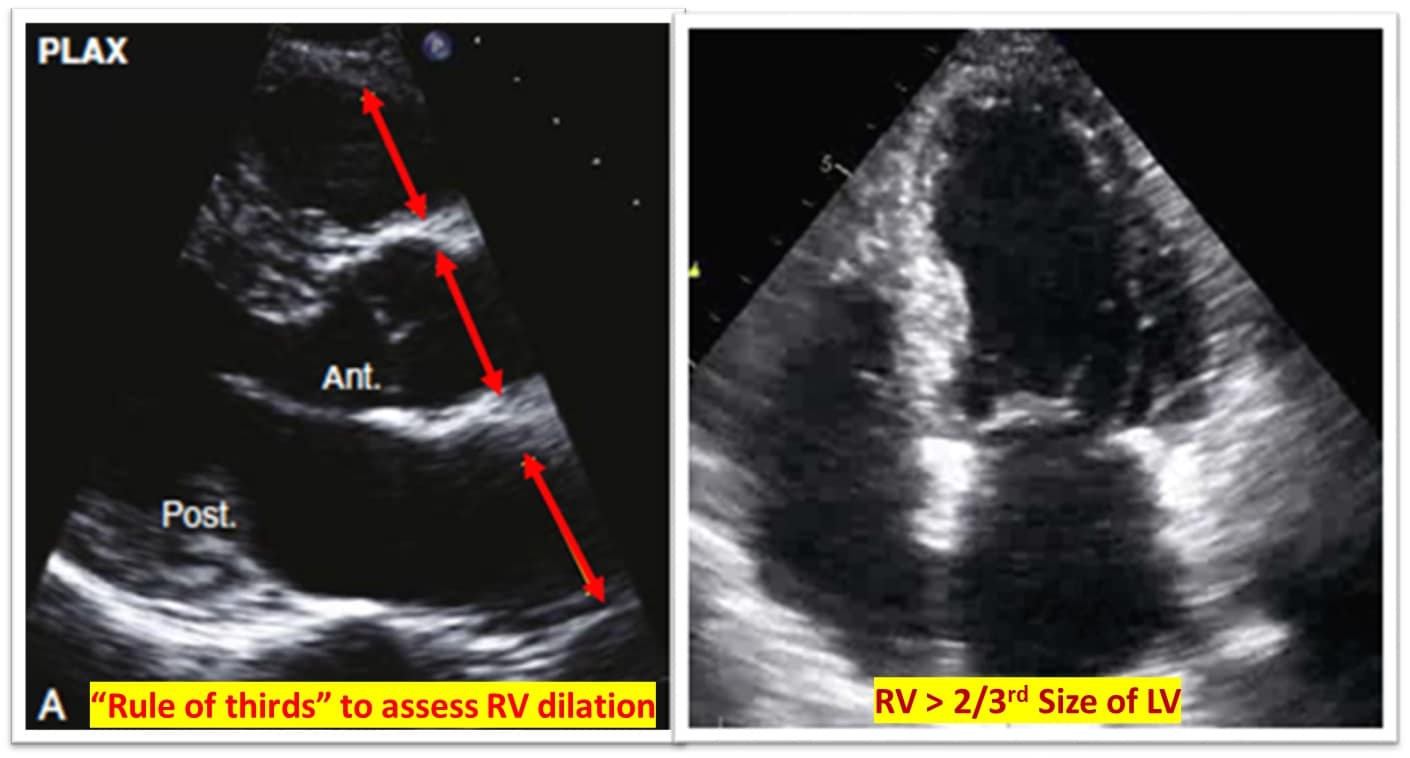
Figure 2: Qualitative RV dimensions.
RV = right ventricle, LV = left ventricle
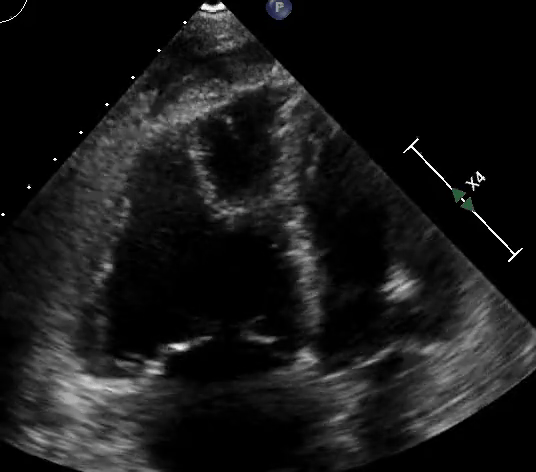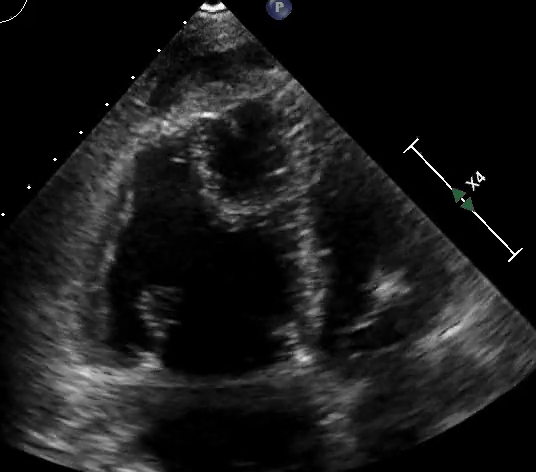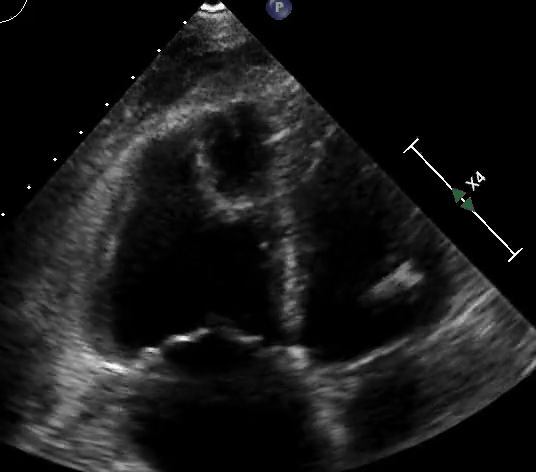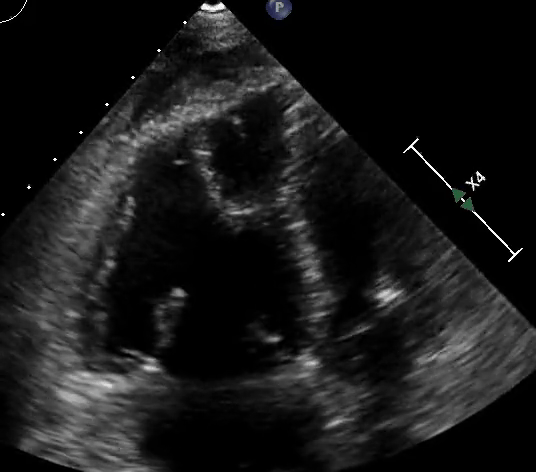
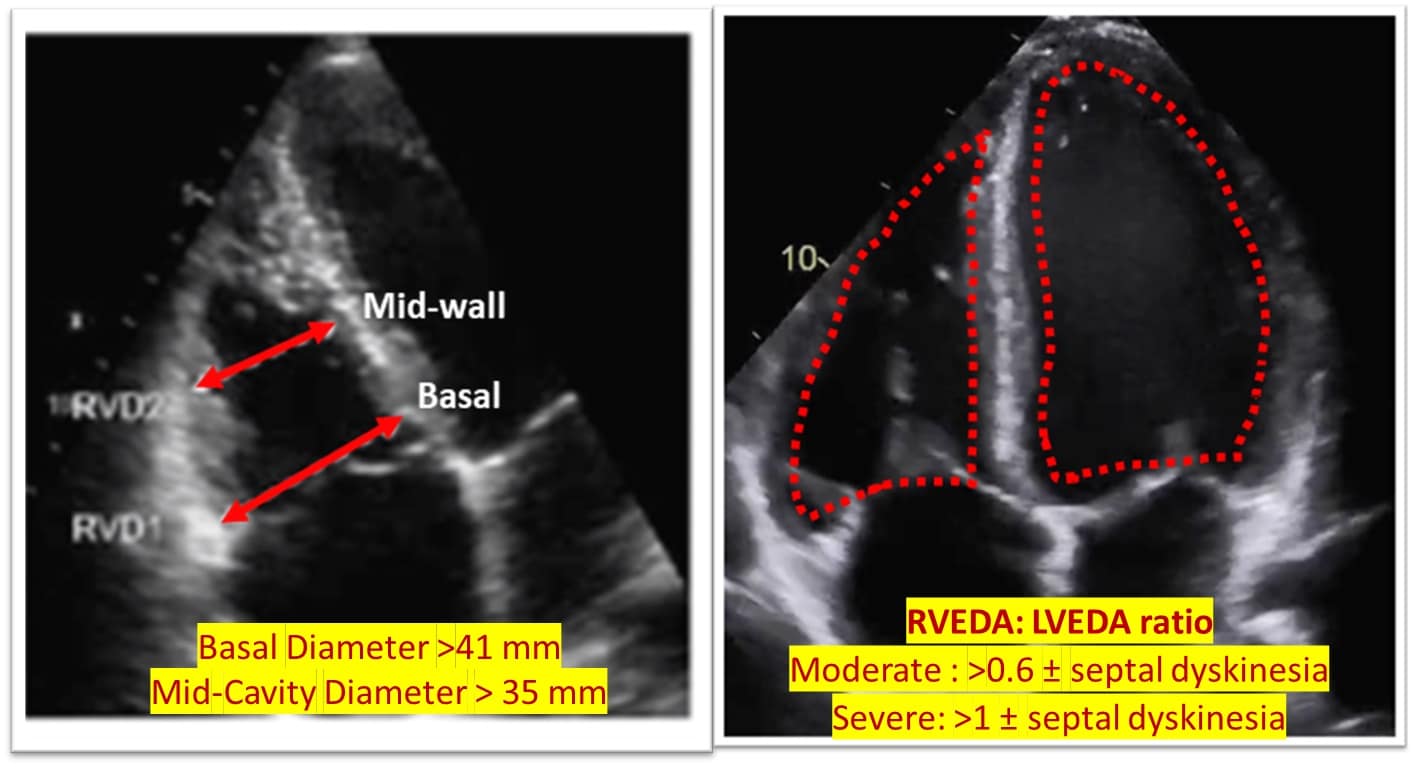
Figure 3: Quantitative RV dimensions.
RVEDA = right ventricular end-diastolic area, LVEDA = left ventricular end-diastolic area
RV free wall thickness was measured in the subcostal view with M mode with > 5 mm, indicating RV hypertrophy, a marker of chronic RV injury.10 Septal kinetics is another key observation. A D-shaped septum instead suggests RV overload with flattening in systole and diastole indicating pressure and volume overload, respectively (Video 5).6
RV Systolic Function Assessment
RV systolic function is assessed using regional measurements like tricuspid annular plane systolic excursion (TAPSE), maximal systolic velocity of the tricuspid annulus (TDI RV S’), or global measurement with RV Fractional area change (FAC), RVFW strain, and RV index myocardial performance (RIMP).10,11
TAPSE is the systolic displacement of the tricuspid annulus towards the RV apex measured at peak systole using M mode. A value of TAPSE < 17 mm is the most validated surrogate for RV systolic dysfunction function due to high inter-rater and intra-rater reliability. 10 An TDI RV S’, another marker routinely used to assess for RV injury, measures the peak systolic myocardial velocity of the tricuspid annulus. TDI RV S’ velocity < 9.5 cm/sec is consistent with RV systolic dysfunction. 8 Alternatively, sTAPSE (subcostal TAPSE) and subcostal echocardiographic assessment of tricuspid annular kick have been suggested for use in patients with challenging apical views.14 (Figure 4)
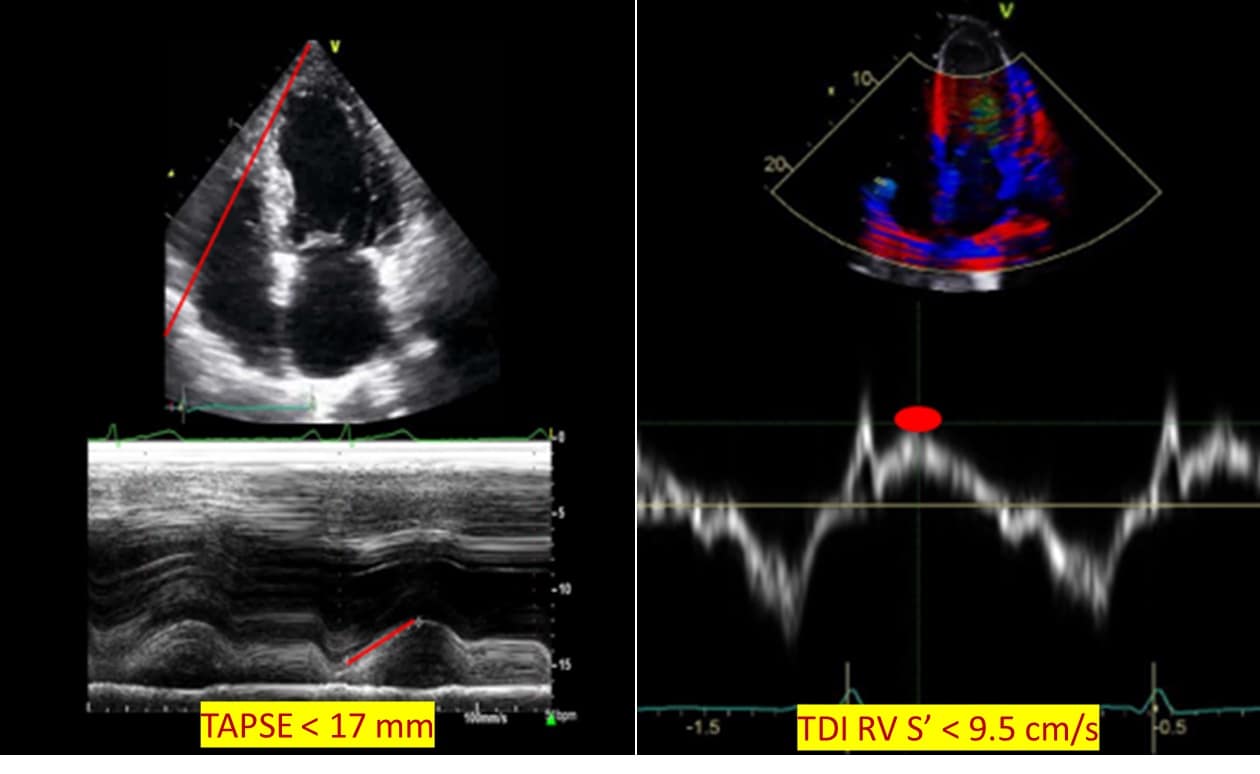
Figure 4: Regional right ventricular systolic function.
TAPSE = tricuspid annular plane systolic excursion, TDI RV S’ = Tissue Doppler imaging: right ventricular lateral tricuspid annulus peak systolic velocity
Overall, RV systolic function can be assessed using RV FAC and RIMP. FAC is measured in an A4C or RV-focused view, the percentage change of RV area at the end of systole from the end of diastole. Importantly, it is not angle-dependent and encompasses both radial and longitudinal function with FAC <35% considered abnormal.15 (Figure 5) RIMP or Tei index, obtained with Doppler at lateral annulus or pulsed wave Doppler at the tricuspid valve, is the ratio of time spent during the non-ejection time and, importantly, independent of the RV’s geometry. Time spent in non-ejection time increases with failure in RV dysfunction. Serial measurements can be used in prognostication of pulmonary hypertension and require a regular R-R interval for accurate assessment, but its utility is limited in critically ill patients.10,16
RVFW strain assesses RV systolic function using speckle tracking to measure the degree of deformation with values less negative than -20% considered abnormal. It is increasingly being used in the assessment of RV in critically ill patients.10,17,18 (Figure 5)
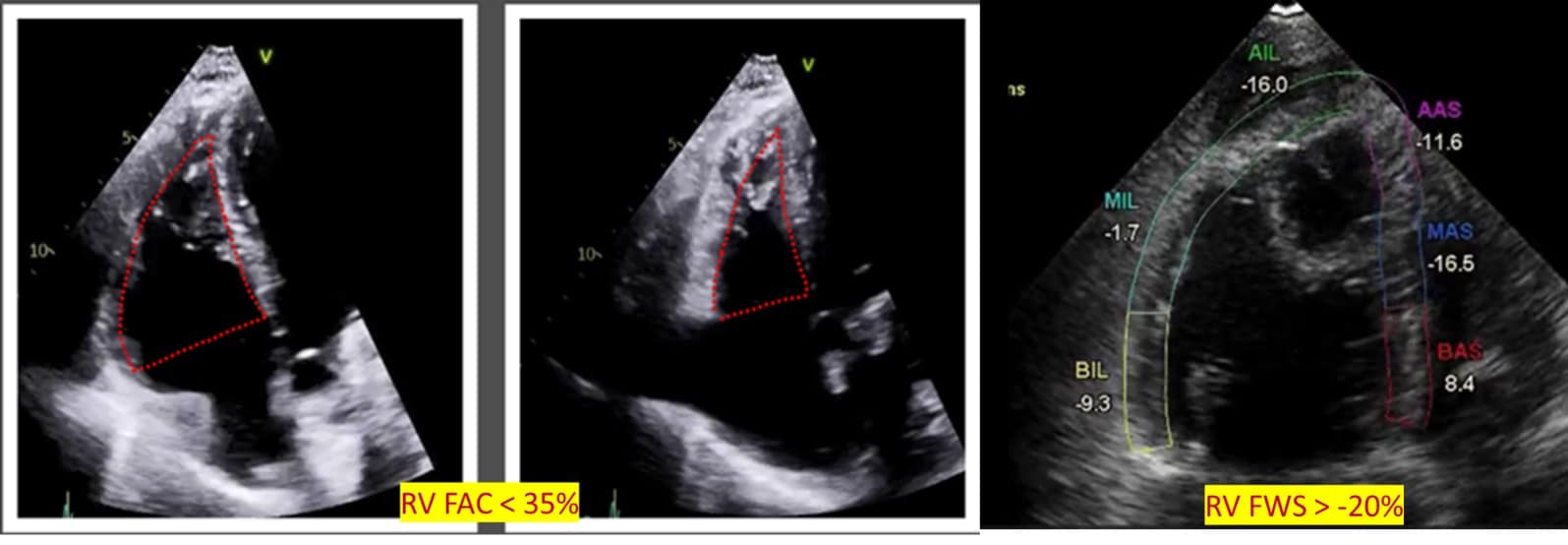
Figure 5: Global right ventricular systolic function.
RV = right ventricle, FAC = fractional area change, FWS = free wall strain
Pulmonary Hemodynamics
Another important assessment that can be performed with an echocardiogram is pulmonary hemodynamics. Right ventricular systolic pressure is calculated by applying the simplified Bernoulli equation to TR jet velocity and added to right atrial pressure [4 (TR jet velocity)2 + RAP]. Right ventricular outflow tract (RVOT) velocity time integral (VTI) is increasingly being used to characterize pulmonary vascular resistance (PVR). The normal RVOT VTI jet has a parabolic velocity curve with a delayed onset to peak flow. As PVR increases, the flow peaks earlier.11,19 Lastly, while pressure-volume loop measurement is the gold standard for RV-pulmonary artery coupling measurements, TAPSE/PASP is increasingly used as a surrogate. A variety of cutoffs for TAPSE/PASP are reported throughout the literature and vary depending on the patient population.20 (Figure 6)
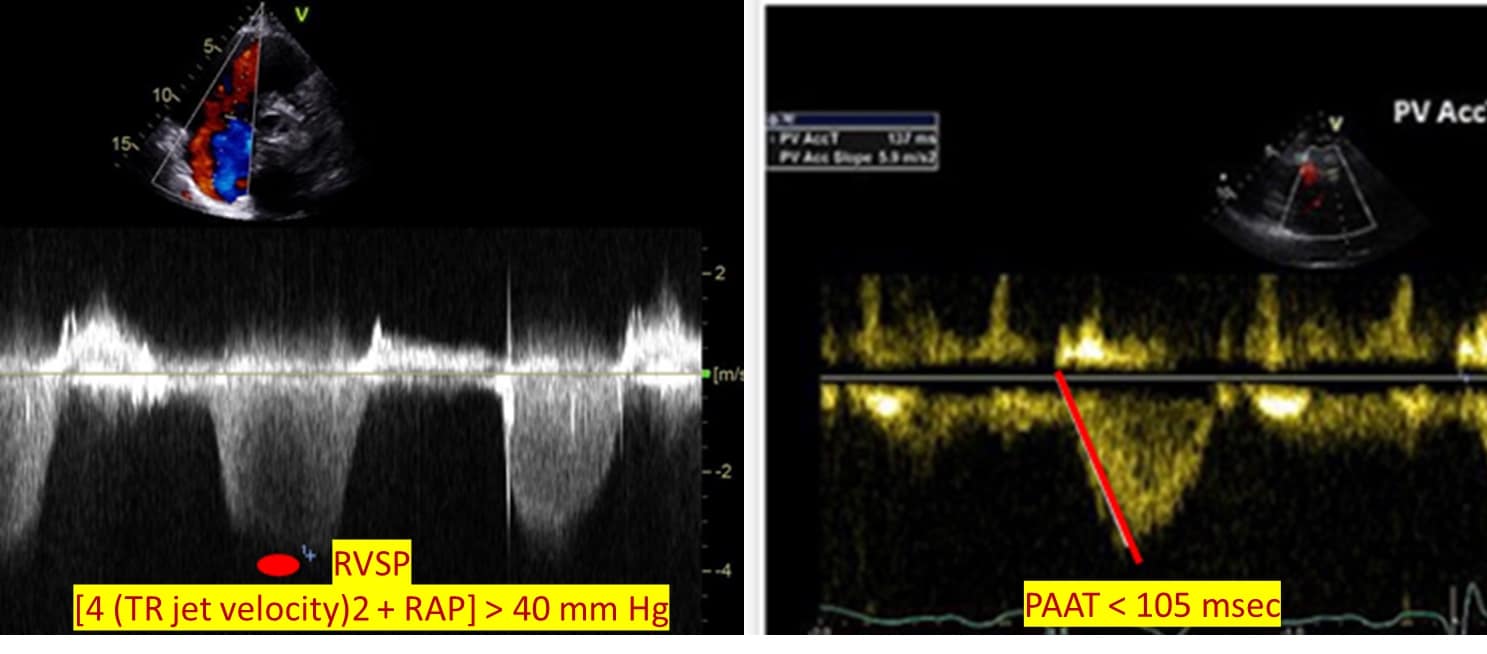
Figure 6: RV-PA hemodynamic assessment.
RVSP = right ventricular systolic pressure, TR = tricuspid regurgitant, RAP = right atrial pressure, PAAT = pulmonary artery acceleration time
Conclusion
The importance of RV dysfunction in critically ill patients is increasingly recognized. Skilled use of point-of-care echocardiography in this population allows for advanced RV function assessment to facilitate RV injury detection and guide management.



References:
- Zochios V, Shelley B, Antonini MV, et al. Mechanisms of acute right ventricular injury in cardiothoracic surgical and critical care settings: part 1. J Cardiothorac Vasc Anesth 2023;37(10):2073-86. https://doi.org/10.1053/j.jvca.2023.06.014
- Yusuff H, Chawla S, Sato R, et al. Mechanisms of acute right ventricular injury in cardiothoracic surgical and critical care settings: part 2. J Cardiothorac Vasc Anesth 2023;37(11):2318-26.https://doi.org/10.1053/j.jvca.2023.07.018
- Vallabhajosyula S, Shankar A, Vojjini R, et al. Impact of right ventricular dysfunction on short-term and long-term mortality in sepsis. Chest 2021;159(6):2254-63. https://doi.org/10.1016/j.chest.2020.12.016
- Sato R, Dugar S, Cheungpasitporn W, et al. The impact of right ventricular injury on the mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care 2021;25(1):172. https://doi.org/10.1186/s13054-021-03591-9
- Anavekar NS, Skali H, Bourgoun M, et al. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (From the Valiant echo study). Am J Cardiol 2008;101(5):607-12. https://doi.org/10.1016/j.amjcard.2007.09.115
- Khemasuwan D, Yingchoncharoen T, Tunsupon P, et al. Right ventricular echocardiographic parameters are associated with mortality after acute pulmonary embolism. J Am Soc Echocardiogr 2015;28(3):355-62. https://doi.org/10.1016/j.echo.2014.11.012
- Kamperidis V, Nihoyannopoulos P, Bax JJ, et al. Assessing the right ventricle. In: Nihoyannopoulos P, Kisslo J, eds. Echocardiography. Springer International Publishing; 2018:373-95. https://10.1007/978-3-319-71617-6_15
- Campbell SJ, Bechara R, Islam S. Point-of-care ultrasound in the intensive care unit. Clin Chest Med 2018;39(1):79-97. https://doi.org/10.1016/j.ccm.2017.11.005
- Dugar S, Sato R, Zochios V, et al. Defining right ventricular dysfunction in acute respiratory distress syndrome. J Cardiothorac Vasc Anesth 2022;36(2):632-4. https://doi.org/10.1053/j.jvca.2021.09.001
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr 2010;23(7):685-713. https://doi.org/10.1016/j.echo.2010.05.010
- Zaidi A, Knight DS, Augustine DX, et al. Echocardiographic assessment of the right heart in adults: a practical guideline from the British Society of Echocardiography. Echo Res Pract 2020;7(1):G19-41. https://doi.org/10.1530/ERP-19-0051
- Genovese D, Mor-Avi V, Palermo C, et al. Comparison between four-chamber and right ventricular–focused views for the quantitative evaluation of right ventricular size and function. J Am Soc Echocardiogr 2019;32(4):484-94. https://doi.org/10.1016/j.echo.2018.11.014
- Orde S, Slama M, Yastrebov K, et al. Subjective right ventricle assessment by echo qualified intensive care specialists: assessing agreement with objective measures. Crit Care 2019;23(1):70. https://doi.org/10.1186/s13054-019-2375-z
- Main AB, Braham R, Campbell D, et al. Subcostal TAPSE: a retrospective analysis of a novel right ventricle function assessment method from the subcostal position in patients with sepsis. Ultrasound J 2019;11(1):19. https://doi.org/10.1186/s13089-019-0134-7
- Anavekar NS, Skali H, Bourgoun M, et al. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (From the Valiant echo study). Am J Cardiol 2008;101(5):607-12. https://doi.org/10.1016/j.amjcard.2007.09.115
- Tei C, Dujardin KS, Hodge DO, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr 1996;9(6):838-47. https://doi.org/10.1016/S0894-7317(96)90476-9
- Bleakley C, de Marvao A, Morosin M, et al. Utility of echocardiographic right ventricular subcostal strain in critical care. Eur Heart J Cardiovasc Imaging 2022;23(6):820-8. https://doi.org/10.1093/ehjci/jeab105
- Lemarié J, Maigrat CH, Kimmoun A, et al. Feasibility, reproducibility and diagnostic usefulness of right ventricular strain by 2-dimensional speckle-tracking echocardiography in ARDS patients: the ARD strain study. Ann Intensive Care 2020;10(1):24. https://doi.org/10.1186/s13613-020-0636-2
- Hockstein MA, Haycock K, Wiepking M, et al. Transthoracic right heart echocardiography for the intensivist. J Intensive Care Med 2021;36(9):1098-1109. https://doi.org/10.1177/08850666211003475
- Schmeisser A, Rauwolf T, Groscheck T, et al. Pressure–volume loop validation of TAPSE/PASP for right ventricular arterial coupling in heart failure with pulmonary hypertension. Eur Heart J Cardiovasc Imaging 2021;22(2):168-76. https://doi.org/10.1093/ehjci/jeaa285