POCUS Spotlight: Lung Ultrasound
Cite as: Manson W, Hogg R. How I do it: lung ultrasound. ASRA Pain Medicine News 2022;47. https://doi.org/10.52211/asra020122.005.
Read this article in Spanish
Lung ultrasound (LUS) is a powerful diagnostic tool for patients with dyspnea, hypotension, ventilation difficulty, or other abnormal vital signs. It can be performed at the patient’s bedside, is repeatable, and provides the clinician with immediate information. Additionally, LUS may be combined with focused cardiac ultrasound to enhance a clinician’s ability to rapidly diagnose and treat patients with critical conditions. Anesthesiologists performing ultrasound-guided regional anesthesia or pain medicine are already familiar with ultrasound equipment, which makes point-of-care ultrasound (POCUS) examination skills easy to acquire.
Conceptually, LUS should be considered as four different exams: pneumothorax, interstitial artifacts, pleural effusion, and diaphragm dysfunction. Each exam yields unique information that can explain abnormal vital signs or symptoms of dyspnea. This article uses the I-AIM format (indication, acquisition, interpretation, and management) to empower anesthesiologists to diagnose patients through LUS.
Indication
Perioperative respiratory compromise has a variety of differential diagnoses, many of which may present with a similar clinical picture. LUS, with its immediate availability at the bedside, can help clinicians expedite a diagnosis and monitor response to clinical interventions. For instance, LUS can be used to diagnose a pneumothorax or diaphragmatic dysfunction immediately following an upper extremity peripheral nerve block.
LUS is particularly sensitive and specific for the diagnosis of pneumothorax1, interstitial syndrome,2 and pleural effusion.3 In fact, Lichtenstein and Mezière demonstrated its ability to rapidly diagnose the cause of hypoxemia in up to 90% of patients.4 LUS can identify diaphragmatic dysfunction, assist with preoperative optimization, diagnose pneumonia, and even aid in early COVID-19 diagnosis.5 LUS is superior to auscultation in the identification of bronchial intubation6 and may be used in combination with cardiac ultrasound for hemodynamic assessment.7
Acquisition
To evaluate patients using LUS, we divide the exam into four components: pneumothorax, interstitial, pleural effusion, and diaphragm function. We use a linear or curvilinear probe with a lung exam preset and direct the indicator cephalad. In situations where pneumothorax is more likely, the linear probe enhances the pleural line’s resolution to visualize lung sliding, but in most clinical situations, we use the curvilinear probe for the entire LUS exam.
We image six lung zones—the anterior, lateral, and posterior chest on the left and right (see Figure 1)—and at least three interspaces in each zone for a total of 18 locations (see Figure 2). There are multiple protocols for LUS, including BLUE,4 FLUID,8 RADiUS,9 and ETUDES,10 but it is more important to image the entire chest than to follow a certain protocol.

Figure 1. Probe position for pneumothorax and interstitial assessment

Figure 2. Anterior, lateral, and posterior areas for lung ultrasound examination
In each area of the chest, we confirm lung sliding and the absence of a lung point with B-mode ultrasound to rule out pneumothorax. We evaluate for B-lines to assess for interstitial syndrome or any situation with increased interstitial fluid or thickness, such as pulmonary edema, COVID-19, acute respiratory distress syndrome (ARDS), transfusion related acute lung injury (TRALI), and pulmonary fibrosis.
We start the pleural effusion evaluation by imaging the right hemithorax in a coronal plane at the posterior axillary line. The liver serves as the acoustic window to visualize the diaphragm. Pleural effusions will appear as an anechoic area cephalad to the diaphragm. We repeat the exam on the left, noting that the relatively small spleen will make the exam more technically challenging.
Diaphragmatic function is most easily assessed at or close to the zone of apposition, where the diaphragm is apposed to the chest wall. Here it is possible to measure the difference in diaphragm muscle thickness at end inhalation and exhalation; impairment is associated with diaphragm dysfunction. As an alternative evaluation for diaphragm function, we place the curvilinear probe in the subcostal region using the liver or spleen as an acoustic window. In this position, we are able to identify the diaphragm’s caudal movement. With forced inspiration, the normally functioning diaphragm rapidly moves in a caudal direction. M-mode can further quantify that motion.
Interpretation
Air in the lung will scatter most ultrasound waves, limiting ultrasound imaging of the normal lung to the pleura. The parietal and visceral pleura sliding across one another is known as lung sliding, shimmering, or “ants on a line.” With M-mode, pleural sliding appears as the seashore sign with a static, linear pattern superficial to the pleural line and a sandy or granular pattern below the pleural line, a result of irregular reflection of sound waves from alveolar tissue (see Figure 3A). A lung pulse occurs in the absence of normal lung ventilation: When a patient’s right main stem is intubated, the pleura on the left chest will pulse with each beat of the heart. Horizontal reverberation artifacts known as A-lines (see Figure 4) may appear in regular, repetitive intervals deep to the pleural line.
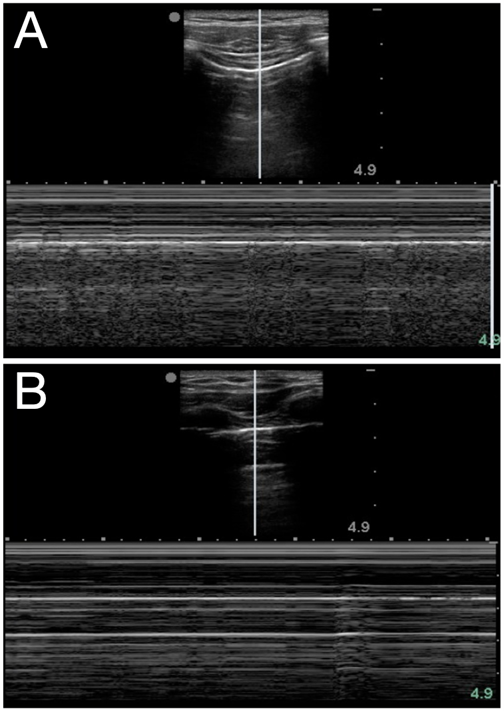
Figure 3. Seashore (A) and stratosphere (B) signs in M-mode

Figure 4. Normal lung ultrasound demonstrating the pleural line and an A-line artifact
A comet-tail artifact is a vertical hyperechoic line that emerges from the pleural line (see Figure 5). Comet tails dissipate toward the far field of the ultrasound image. In contrast, B-lines, which are three or more comet-tail artifacts, extend to the far field of the image (see Figure 6). B-lines in the anterior chest almost always represent pathology, whereas B-lines at the most dependent locations may be a normal finding. Depending on the underlying cause, the lines may be homogenous, irregularly spaced, localized, or disseminated. Although B-lines are most commonly seen with pulmonary edema, they are also present in any disease that increases lung density, including pneumonia, pulmonary fibrosis or infarction. Because of disruption of normal alveolar composition, A-lines are not visible in these areas.

Figure 5. Comet tail artifact

Figure 6. Multiple B-lines
Pneumothorax
LUS Identification of a pneumothorax should be performed in a stepwise manner, elucidating whether signs are present or absent. At each rib space, identify the pleural line and ask: Is there sliding? Can you see a lung pulse? Are A-lines present? If the answer to any of those questions is no, then look for a lung point, or the junction of the presence and absence of normal lung sliding. The lung point occurs where the visceral and parietal pleura are no longer contiguous, and it is a very specific finding for a pneumothorax. If lung sliding is unclear, M-mode can further differentiate: The normal seashore sign is replaced with that of a stratosphere or “barcode” sign (see Figure 3B) that may represent a pneumothorax.
Interstitial Syndrome
The interstitial syndrome represents any disease process that results in increased density of the interstitial space between alveoli, such as pulmonary edema, pneumonia, ARDS, TRALI, COVID-19, or pulmonary fibrosis. The number and distribution of B-lines will further define the specific cause of the interstitial syndrome. For instance, B-lines separated by at least 7 mm will frequently be caused by pulmonary edema, whereas those separated by less than 3 mm will frequently be caused by alveolar flooding or ground glass opacities.
Pleural Effusion
The liver and spleen serve as acoustic windows to assess for pleural effusion. With breathing, a normal aerated lung passes in front of the probe, obliterating visualization of the diaphragm, liver, and spleen. Called the curtain sign, it negates the possibility of significant effusion. LUS is more sensitive to small volumes of fluid compared to chest x-ray; in the thoracic cavity, fluid appears as a dark, hypoechoic area cephalad to the diaphragm. Atelectatic lung may be seen as a wedge-shaped mass in a large effusion, freely moving with the respiratory cycle. Our personal favorite nickname for the phenomenon is the “lung monster” sign, so termed because of the mobile shapes made by the unopposed lung tissue. The spine sign is visualization of the thoracic vertebral bodies cephalad to the diaphragm, which is present only with pleural effusion or hemothorax because of the removal of air impedance (see Figure 7).

Figure 7. Pleural effusion in left hemithorax
Diaphragm Dysfunction
Anesthesiologists can use LUS to assess diaphragm function through multiple signs. At the zone of apposition, the diaphragm at end inspiration should thicken by 30% or more compared to end expiration. Cephalad motion of the diaphragm during inhalation is referred to as paradoxical motion of the diaphragm and is consistent with hemidaphragmatic paresis. Other findings consistent with hemidiaphragmatic paresis include excursion of less than 4 mm or a difference of greater than 50% between the excursion of one hemidiaphragm compared to the other.11
LUS can offer clinicians additional diagnostic signs and information that are not discussed in this limited article, including the C-lines, shred sign, lung hepatization, and air bronchograms. See Table 1 and the article “Lung Ultrasound for the Regional Anesthesiologist and Pain Physician” for more information.12
Table 1. Glossary of common terminology used in lung ultrasound
| Terminology | Definition |
|---|---|
| A-lines | Horizontal reverberation artifacts that occur beneath the pleural line at regular intervals equal to the distance between the ultrasound probe and pleura |
| Acoustic window | Structures in the near field of the ultrasound view that promote ultrasound wave transmission. The liver and spleen are common acoustic windows to the lung bases for pleural effusion or diaphragmatic function diagnosis. |
| Air bronchogram | Easily recognizable pattern of air-filled bronchi surrounded by fluid filled alveoli; may be dynamic (eg, hyperechoic movement of air in consolidated lung tissue) or static (eg, severe atelectasis) |
| B-lines | Three or more comet-tail artifacts that extend to the far field of the ultrasound view; may be a normal finding in dependant areas. B-lines are associated with the interstitial syndrome (ie, increased fluid or thickness of the interstitial space between alveoli) and pathologies such as pulmonary edema, consolidation, acute respiratory distress syndrome, transfusion related acute lung injury, and pulmonary fibrosis. |
| Seashore sign | Classic M-mode appearance of normal lung where the “sea” is the relatively immobile chest wall tissue and muscle layers and the “sandy beach” is pleural sliding (see Figure 3A) |
| Stratosphere sign | Also known as the barcode sign; suggestive of pneumothorax when the seashore sign’s sandy beach is replaced by static parietal pleura and a repetitive linear appearance on M-mode (see Figure 3B) |
| C-lines | Basal comet-tail artifacts generated by consolidated lung tissue |
| Comet-tail artifact | Reverberation artifact seen in normal lung as a vertical hyperechoic line emerging from the pleural line and dissipating toward the far field of the ultrasound view; differs from B-lines, which extend to the edge of the image |
| Curtain sign | Dynamic artifact at the showing the cranio-caudad movement of normally aerated lung in front of the probe at the fully aerated lung base; reduced or completely absent in lung base pathology such as pleural effusion, atelectasis, and consolidation |
| Lung hepatization | Sign of significant consolidation, where lung tissue appears isoechoic (ie, similar sonographic appearance) compared with liver parenchyma |
| Lung monster | Wedge-shaped mass of atelectatic lung in a large effusion that moves freely with the respiratory cycle |
| Lung pulse | Transmission of cardiac pulsation to lung, causing the pleural line to move in synchronicity with the heartbeat; occurs with an absence of ventilation of normal lung tissue (eg, right mainstem intubation) and is absent in pneumothorax |
| Lung sliding | Parietal and visceral pleura sliding across one another; absent in pneumothorax, where intrathoracic air separates the visceral and parietal pleura, and may be absent following pleurodesis |
| Mirror artifact | Artifacts caused when the ultrasound beam encounters a strong and smooth reflector, such as the diaphragm, creating a false image; absent in pleural effusion |
| Shred sign | Interface of fully aerated and consolidated lung tissue creating an irregular, static hyperechoic line |
| Spine sign | Pleural effusion–associated visualization of the thoracic vertebra, which are normally impeded by aerated lung above the diaphragm |
| Zone of apposition | Area of the diaphragm parallel to the chest wall, at the caudal aspect of the chest and just cephalad to the diaphragm’s insertion on the costal cartilages, where the parietal pleural of the chest and the diaphragmatic pleura are in direct apposition without lung tissue in between |
Management
Anesthesiologists must use clinical context to manage patients with abnormal LUS findings. A comfortable patient with a small pneumothorax is managed very differently from one with a tension pneumothorax under general anesthesia. Similarly, multiple lung fields with B-lines may be normal in a patient with pulmonary fibrosis but not in a dyspneic postoperative patient following massive transfusion. The key is to incorporate the LUS profile into the patient’s clinical scenario and reassess if the clinical scenario changes.
Pearls and Pitfalls
- Use a curvilinear probe for the entire exam to avoid repeated probe exchanges. In our practice, we only rarely use a linear probe, usually in the setting of high suspicion of a pneumothorax.
- Focal B-lines are more suggestive of consolidation or atelectasis. Widespread, diffuse B-lines are more suggestive of pulmonary edema.
- Extend the BLUE protocol to include scanning medial to the scapula because the upright position will assist with visualization.13 This is of particular benefit in COVID-19 diagnosis and to define the magnitude of pleural effusion.
- Mirror artifacts (see Figure 8) can be misleading and prompt an incorrect consolidation diagnosis, especially when they appear on the right-hand side. They are caused when the ultrasound beam encounters a strong and smooth reflector, such as the diaphragm, creating a false image behind the reflector. The presence of mirror artifacts eliminates the possibility of pleural effusion.
- Many patients are technically challenging to image because body habitus or positioning during surgery. Using a curvilinear probe and regularly practicing in those types of situations will improve your image acquisition.

Figure 8. Mirror artifact
In conclusion, LUS is a powerful tool for regional anesthesiologists and pain physicians. Building on the basics of lung ultrasound and its strengths and weaknesses presented in this article and understanding the subtle changes of the pleural, pleural artifacts, pleural effusions, and diaphragm function will help clinicians treat critical patients.
William C. Manson, MD, is the medical director of perioperative medicine and an assistant professor of anesthesiology at UVA Health in Charlottesville, VA.

Rosie M. Hogg, MB, ChB, FRCA, MD, is a consultant anaesthetist for Belfast Health and Social Care Trust in Belfast, United Kingdom.
References
- Alrajab S, Youssef AM, Akkus NI, et al. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17(5):R208. https://doi.org/10.1186/cc13016
- Dubinsky TJ, Shah H, Sonneborn R, Hippe DS. Correlation of B-lines on ultrasonography with interstitial lung disease on chest radiography and CT imaging. Chest. 2017;152(5):990–8. https://doi.org/10.1016/j.chest.2017.05.003
- Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran). 2016;4(1):1–10. https://pubmed.ncbi.nlm.nih.gov/26862542
- Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol [published correction appears in Chest. 2013;144(2):721]. Chest. 2008;134(1):117–25. https://doi.org/10.1378/chest.07-2800
- Haskins SC, Bronshteyn Y, Perlas A, et al. American Society of Regional Anesthesia and Pain Medicine expert panel recommendations on point-of-care ultrasound education and training for regional anesthesiologists and pain physicians—part I: clinical indications. Reg Anesth Pain Med 2021; 46(12):1031–47. https://doi.org/10.1136/rapm-2021-102560
- Ramsingh D, Frank E, Haughton R, et al. Auscultation versus point-of-care ultrasound to determine endotracheal versus bronchial intubation: a diagnostic accuracy study. Anesthesiology. 2016;124(5):1012–20. https://doi.org/10.1097/aln.0000000000001073
- Lichtenstein D, Karakitsos D. Integrating lung ultrasound in the hemodynamic evaluation of acute circulatory failure (the fluid administration limited by lung sonography protocol). J Crit Care. 2012;27(5):533.e11–9. https://doi.org/10.1016/j.jcrc.2012.03.004
- Chiem AT, Chan CH, Ander DS, Kobylivker AN, Manson WC. Comparison of expert and novice sonographers’ performance in focused lung ultrasonography in dyspnea (FLUID) to diagnose patients with acute heart failure syndrome. Acad Emerg Med. 2015;22(5):564–73. https://doi.org/10.1111/acem.12651
- Manson W, Hafez N. The rapid assessment of dyspnea with ultrasound: RADiUS. Ultrasound Clinics. 2011;6(2):261–76. http://doi.org/10.1016/j.cult.2011.03.010
- Liteplo AS, Marill KA, Villen T, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): sonographic B-lines and N-terminal pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med. 2009;16(3):201–10. https://doi.org/10.1111/j.1553-2712.2008.00347.x
- Fayssoil A, Behin A, Ogna A, et al. Diaphragm: pathophysiology and ultrasound imaging in neuromuscular disorders. J Neuromuscul Dis. 2018;5(1):1–10. https://doi.org/10.3233/JND-170276
- Haskins SC, Tsui BC, Nejim JA, Wu CL, Boublik J. Lung ultrasound for the regional anesthesiologist and acute pain specialist. Reg Anesth Pain Med. 2017;42(3):289–98. https://doi.org/10.1097/AAP.0000000000000583
- Havelock T, Teoh R, Laws D, Gleeson F, BTS Pleural Disease Guideline Group. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):61–76. https://doi.org/10.1136/thx.2010.137026

Leave a commentOrder by
Newest on top Oldest on top