Examining Advanced Modalities for Thoracic Epidural Catheter Placement
Epidural analgesia is a popular method of postoperative pain control and is particularly useful when used as part of a multimodal pain medication regimen. However, epidural catheter failure is a frequent problem. Lack of a uniform outcome measure results in heterogeneous estimates of thoracic epidural failure rates, but reported rates range from 34% for overall failure to 13% for technical failure alone.[1-2]
“The role of additional modalities to confirm or facilitate epidural placement may be of notable benefit.”
Technical failure is a particular challenge for the teaching physician who must facilitate a trainee's practice-based learning of a procedure for which tactile feedback is relied on to confirm correct epidural placement.[3] The role of additional modalities to confirm or facilitate epidural placement may be of notable benefit in this setting. This requires broadening our current skill set in an attempt to improve the success of thoracic epidural placement and thereby provide more reliable pain control.
Traditional methods of ensuring entry into the epidural space include loss of resistance (LOR) to air/fluid-filled syringe, hanging drop technique, and fluid column drop (drip method).[4] Of these traditional bedside techniques, loss of resistance to air and/or saline was overwhelmingly the most popular method used by those surveyed.[5] This is likely secondary to the fact that it is generally easily taught, gives decent tactile and visual feedback, and can be done efficiently in a cost-effective manner. Despite these advantages, it is still affected by significant false-positive rates (17%).[6-7]
In an attempt to improve the failure rates for epidural placement, more advanced methods of analyzing epidural catheter placement have been described (Table 1). These approaches can demonstrate not only entry into the epidural space, but also other properties of the newly placed catheter that may help predict its effectiveness in providing analgesia. These include epidural waveform analysis, nerve stimulation, ultrasonography, and fluoroscopy.
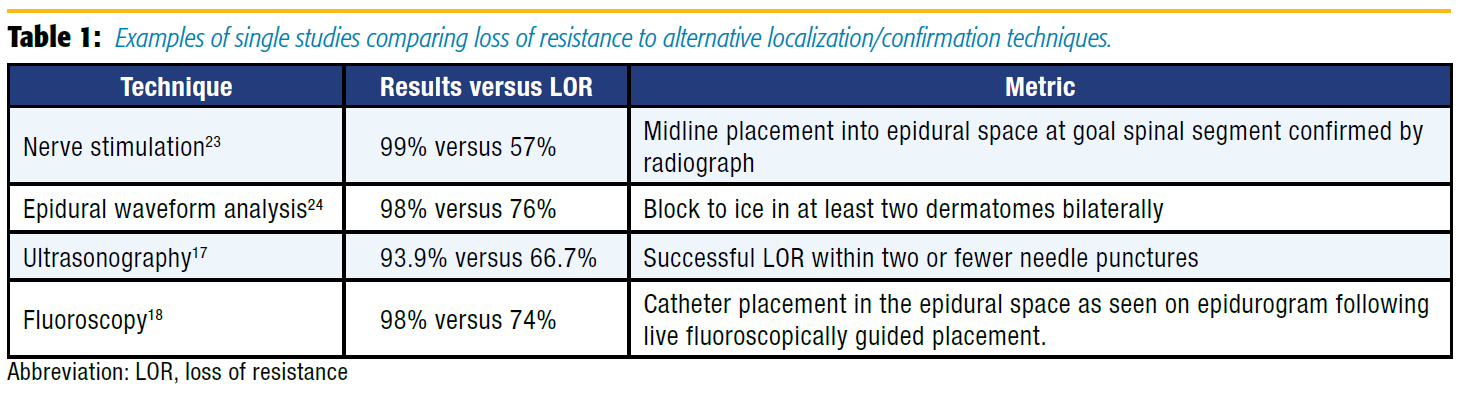
Epidural pressure waveform analysis has become a topic of study following the observation that a drop of saline hanging from the hub of a needle pulsated synchronously with the heart beat once the needle was in the epidural space.[8] It is theorized that this pulsation is a dampened waveform originating in the pulsating spinal cord and conducted through the cerebrospinal fluid and the dura mater to the epidural space.[9-10] Epidural waveform analysis can provide a simple and low cost confirmation of LOR by connecting a sterile pressure transducer to our standard operating room monitors. Leurcharusmee et al11 conducted a blinded observational study of patients undergoing thoracic epidural placement and found a 91.1% sensitivity and 83.8% specificity with this technique. An earlier study conducted by de Medicis et al[12] in patients undergoing lumbar and thoracic epidurals had a lower sensitivity of 81%; however, they studied the pressure waveform through the catheter, which may be less accurate. This technique can be performed quickly with equipment that is already readily available in operating rooms at low cost and minimal time.
Nerve stimulation via the epidural catheter has proven to be beneficial in confirmation of catheter placement into the epidural space. Tsui et al[13] demonstrated improvement in catheter placement confirmation and predicted function. Since that time, the Tsui Test has been described for use in postoperative analgesia, pediatric setting, chronic pain, and obstetric anesthesia. Subsequent studies have verified its high rate of sensitivity and specificity since its description.[7] Advantages include the ability to determine the spinal level of the epidural tip as well as intrathecal, subdural, and intravascular detection. One drawback may be that a specialized catheter is necessary when using bipolar electrical stimulation. However, the technique described by Tsui et al[14] uses monopolar stimulation, which can be performed with commonly available epidural catheters. Additionally, patient discomfort should be considered before performing this technique.
Regional anesthesiologists are increasingly adept at the use of ultrasonography. Therefore, using ultrasonography to assist with neuraxial techniques is a natural progression for many regionalists. Preprocedural ultrasound scanning provides reliable and accurate information on several critical aspects needed for successful epidural placement, such as the interspace level, the midline of the spine, the window between spinous processes/laminae, and depth to ligamentum flavum/dura.[15] In 2002, Grau et al[16] observed that women who received labor epidurals with ultrasound assistance had fewer attempts, more complete analgesia, and improved pain scores as compared to the LOR-only group.
Although most of the published literature thus far has focused on the obstetric population, there has been an increased use of ultrasound guidance for thoracic epidural placement. A recent study evaluating thoracic epidural placement demonstrated no significant decrease in procedure time, but did report a reduction in pain scores in the postanesthesia care unit (PACU) and number of needle puncture sites.[17] It is important to note that all patients studied had a mean age of 58 years and body mass index of 27 kg/m2 . Considering the current thoracic data, there may be less benefit to those with low predicted difficulty. The additional time and skill required for ultrasound-assisted placement may be warranted in patients with known or anticipated difficult epidural placement because of body habitus or spinal abnormalities. Future advancements making real-time ultrasound visualization of Tuohy needle advancement more logistically feasible may dramatically improve the utility of ultrasonography in varied patient populations.
Finally, the use of fluoroscopy for catheter placement and confirmation of catheter tip position has demonstrated not only decreased failure rates but also improved patient outcomes.[18] Realtime fluoroscopic guidance allows visualization of the predicted spread of infusate by examining the pattern of dye spread on epidurogram. The improvement in catheter tip location with this technique has been associated with reduced PACU and hospital length of stay and improved pain scores.[18-19] The downside to this technique is the equipment, financial, and personnel resources required to use fluoroscopy as well as the risk of radiation exposure. These limitations have prevented fluoroscopy from becoming standard practice in the perioperative setting. However, this modality can be of great benefit in patients for whom epidural placement is known or anticipated to be difficult.
Additional, novel techniques and devices are being described that may have potential clinical applications in the future. A real-time, 3D ultrasound rendering technique with needle guide has been developed and is undergoing preliminary tests in humans.[20] A mobile optical probe mounted inside a standard epidural needle has also been developed that alarms once the tissue within the epidural space is detected. This device has thus far been tested only in animals.[21] A small ultrasound transducer, inserted into a Tuohy needle, has been used in a porcine model to detect dura mater and the epidural space.[22]
Although traditional methods of thoracic epidural catheter placement are generally simple and easily taught, the above modalities can be useful adjuncts. Financial and time constraints may dictate that some modalities are reserved for especially difficult cases, but their use should still be considered on a case-by-case basis. As evidence grows for these techniques in varied clinical circumstances, certain ones may be adopted as a standard modality, especially if they are low cost and simple. In the meantime, having the knowledge and skills to use these techniques provides the clinician with additional tools when traditional methods fail, potentially improving patients' analgesia and outcomes.
References
- Hermanides J, Hollmann MW, Stevens MF, Lirk P. Failed epidural: causes and management. Surv Anesthesiol 2013;57:43. doi: 10.1097/01. sa.0000424242.67406.ab.
- McLeod G, Davies H, Munnoch N, Bannister J, MacRae W. Postoperative pain relief using thoracic epidural analgesia: outstanding success and disappointing failures. Anaesthesia 2001;56:75–81.
- Johnson T. Counterbalancing clinical supervision and independent practice: case studies in learning thoracic epidural catheter insertion. Br J Anaesth 2010;105:772–776. doi: 10.1093/bja/aeq233.
- Michel MZ, Lawes EG. Identification of epidural space by drip method. Reg Anesth Pain Med 1991;16(4):236–239.
- Wantman A, Hancox N, Howell PR. Techniques for identifying the epidural space: a survey of practice amongst anaesthetists in the UK. Anaesthesia 2006;61:370–375. doi: 10.1111/j.1365-2044.2006.04534.x.
- Sharrock NE. Recordings of, and an anatomical explanation for, false positive loss of resistance during lumbar extradural analgesia. Br J Anaesth 1979;51(3):253–258.
- Tran DQ, Gonzalez AP, Bernucci F, Finlayson RJ. Confirmation of loss-ofresistance for epidural analgesia. Reg Anesth Pain Med 2015;40:166–173. doi: 10.1097/AAP.0000000000000217.
- Aldrete J, Auad O, Gutierrez V, Wright A. Alberto Gutierrez and the hanging drop. Reg Anesth Pain Med 2005;30:397–404.
- Ghia J. Confirmation of location of epidural catheters by epidural pressure waveform and computed tomography cathetergram. Reg Anesth Pain Med 2001;26:337–341.
- Lennox PH, Umedaly HS, Grant RP, et al. A pulsatile pressure waveform is a sensitive marker for confirming the location of the thoracic epidural space. J Cardiothorac Vasc Anesth 2006;20:659–663. doi: 10.1053/j. jvca.2006.02.022.
- Leurcharusmee P, Arnuntasupakul V, Chora De La Garza D, et al. Reliability of waveform analysis as an adjunct to loss of resistance for thoracic epidural blocks. Reg Anesth Pain Med 2015;40:694–697. doi: 10.1097/ AAP.0000000000000313.
- de Medicis E, Tetrault JP, Martin R, Robichaud R, Laroche L. A prospective comparative study of two indirect methods for confirming the localization of an epidural catheter for postoperative analgesia. Anesth Analg 2005;101:1830– 1833. doi: 10.1213/01.ANE.0000184130.73634.BE.
- Tsui B, Gupta S, Finucane B. Confirmation of epidural catheter placement using nerve stimulation in obstetric patients: the tsui test. Reg Anesth Pain Med 1998; 24:17–23.
- Tsui B, Finucane B. Verifying accurate placement of an epidural catheter tip using electrical stimulation. Anesth Analg 2002;94:1670. doi: 10.1213/00000539-200206000-00063.
- Balki M. Locating the epidural space in obstetric patients-ultrasound a useful tool: continuing professional development. Can J Anaesth 2010;57:1111–1126. doi: 10.1007/s12630-010-9397-y.
- Grau T, Leipold RW, Conradi R, Martin E, Motsch J. Efficacy of ultrasound imaging in obstetric epidural anesthesia. J Clin Anesth 2002;14:169–175.
- Auyong DB, Hostetter L, Yuan SC, Slee AE, Hanson NA. Evaluation of ultrasoundassisted thoracic epidural placement in patients undergoing upper abdominal and thoracic surgery: a randomized, double-blind study. Reg Anesth Pain Med 2017;42:204–209. doi: 10.1097/AAP.0000000000000540.
- Parra MC, Washburn K, Brown JR, et al. Fluoroscopic guidance increases the incidence of thoracic epidural catheter placement within the epidural space: a randomized trial. Reg Anesth Pain Med 2017;42:17–24. doi: 10.1097/ AAP.0000000000000519.
- Yeager MP, Bae EE, Parra MC, et al. Fluoroscopy-assisted epidural catheter placement: an exploratory analysis of 303 pre-operative epidurograms. Acta Anaesthesiol Scand 2016;60:513–519. doi: 10.1111/aas.12649.
- Beigi P, Malenfant P, Rasoulian A, et al. Three-dimensional ultrasound-guided real-time midline epidural needle placement with epiguide: a prospective feasibility study. Ultrasound Med Biol 2017;43:375–379. doi: 10.1016/j. ultrasmedbio.2016.08.033.
- Gong CS, Lin SP, Mandell MS, et al. Portable optical epidural needle—a CMOSbased system solution and its circuit design. PLoS One 2014;9:e106055. doi: 10.1371/journal.pone.0106055.
- Chiang HK, Zhou Q, Mandell MS, et al. Eyes in the needle: novel epidural needle with embedded high-frequency ultrasound transducer—epidural access in porcine model. Anesthesiology 2011;114:1320–1324. doi: 10.1097/ ALN.0b013e31821b5746.
- Hayatsu K, Tomita M, Fujihara H, et al. The placement of the epidural catheter at the predicted site by electrical stimulation test. Anesth Analg 2001;93:1035– 1039.
- Arnuntasupakul V, Van Zundert TC, Vijitpavan A, et al. A randomized comparison between conventional and waveform-confirmed loss of resistance for thoracic epidural blocks. Reg Anesth Pain Med 2016;41:368–373. doi: 10.1097/ AAP.0000000000000369.
Leave a commentOrder by
Newest on top Oldest on top